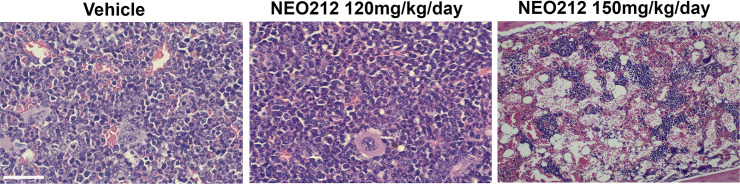
Fig 11. Bone marrow toxicity following dose escalation studies with NEO212.
NEO212 appears to be well tolerated by the animals with no bone marrow toxicity observed at clinically relevant doses (i.e., 50 mg/kg/day) given orally over 2 weeks (using a schedule of administration of 5 days on/2 days off). Significant changes in bone marrow cellularity and morphology by H&E staining are observed in animals dosed with NEO212 at 150 mg/kg/day, but not for doses up to 120 mg/kg/day.