Fig 1.
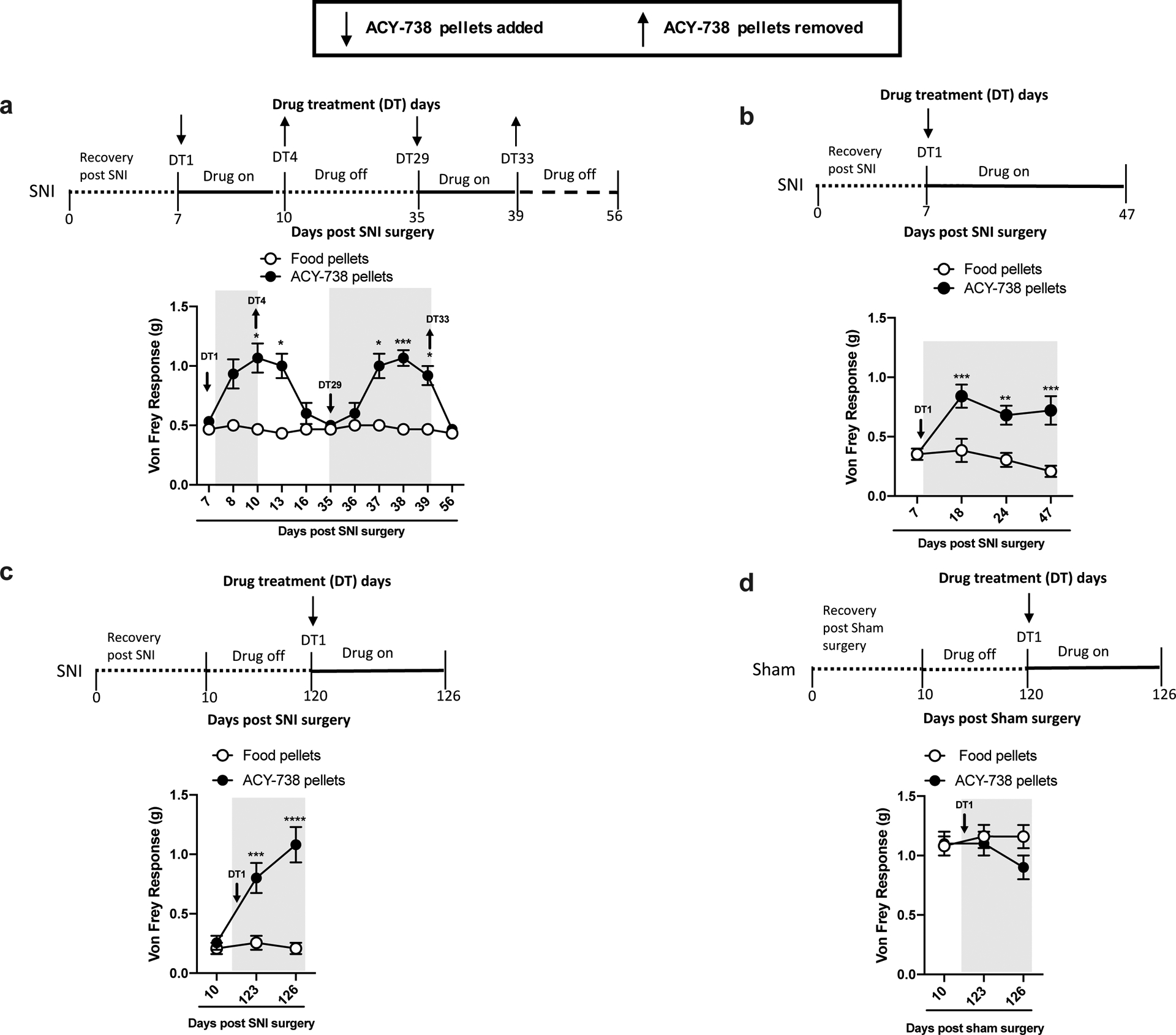
ACY-738 alleviates mechanical allodynia in the SNI model of peripheral nerve injury. Down arrows indicate when ACY-738 pellets were added, and up arrows indicate when ACY-738 pellets were removed. Shaded areas on figures indicate the duration of drug treatment. A significant interaction between day of measurement x treatment was followed by a Holm-Sidak post hoc test. (a) Mice treated with ACY-738 pellets show reduced mechanical allodynia in the Von Frey assay, compared to control mice treated with regular food pellets. Numbers on arrows indicate the day of drug treatment (DT) relative to drug treatment initiation (DT1). Drug treatment started immediately after SNI baseline (7 days after surgery) measurement (n=6, two-way ANOVA followed by Holm—Sidak’s post hoc test, F(10,99)=8.57, *P<0.05, **P<0.01, ***P<0.001). (b) Long-term treatment with ACY-738 does not lead to tolerance, as mice show the same antiallodynic response throughout the 47 days of treatment. Number on down arrow indicates the day of drug treatment (DT) initiation. Drug treatment started the same day after SNI baseline measurement (7 days after surgery, n=5, two-way ANOVA followed by Holm—Sidak’s post hoc test, F(3,24)=4.13, *P<0.05, **P<0.01, ***P<0.001). (c) Mice under long-term SNI (4 months) show significant reduction of mechanical allodynia when treated with ACY-738 pellets. Control animals received regular food pellets. Number on down arrow, indicates the day of drug treatment (DT) initiation. Drug administration started 4 months (day 120) after the induction of SNI (n=5, two-way ANOVA followed by Holm—Sidak’s post hoc test, F(2,14)=13.31, ***P<0.001, ****P<0.01). (d) ACY-738 pellets have no-effect on sham-operated mice (n=5), two-way ANOVA. Sham surgeries were performed at the same time as SNI. Number on down arrow indicates the day of drug treatment (DT) initiation.
