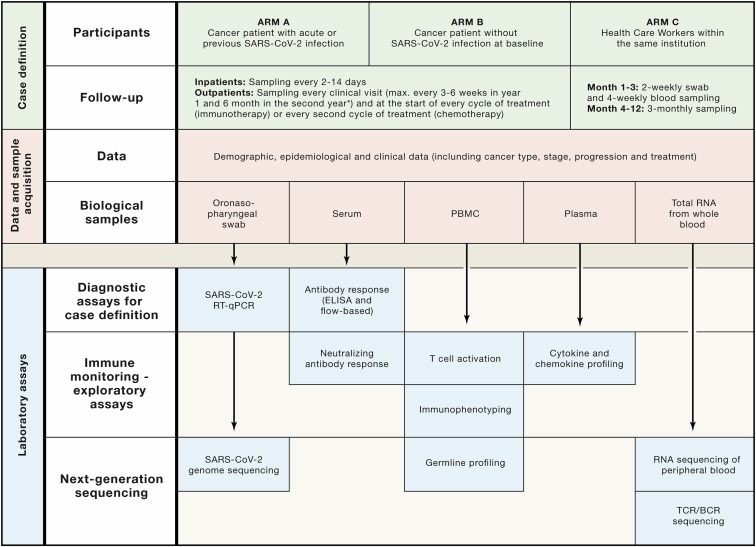
Figure 2.
Overview of CAPTURE Study Procedures and Analysis Plans
Patients are recruited into study arms A or B, and follow-up schedules are bespoke to their COVID-19 status and accounts for their clinical visit/treatment requirements; while healthcare workers are recruited and followed-up according to schedule as outlined. Comprehensive demographic, epidemiological, and clinical data will be collected along with swabs and blood samples. Diagnostic assays will be performed for all participants and will inform selection of samples for detailed immune monitoring and next-generation sequencing. Standard operating procedure documents (for local sample collection, processing, and storage) will be implemented in additional institutions, and sample analyses will be centralized to ensure standardization.