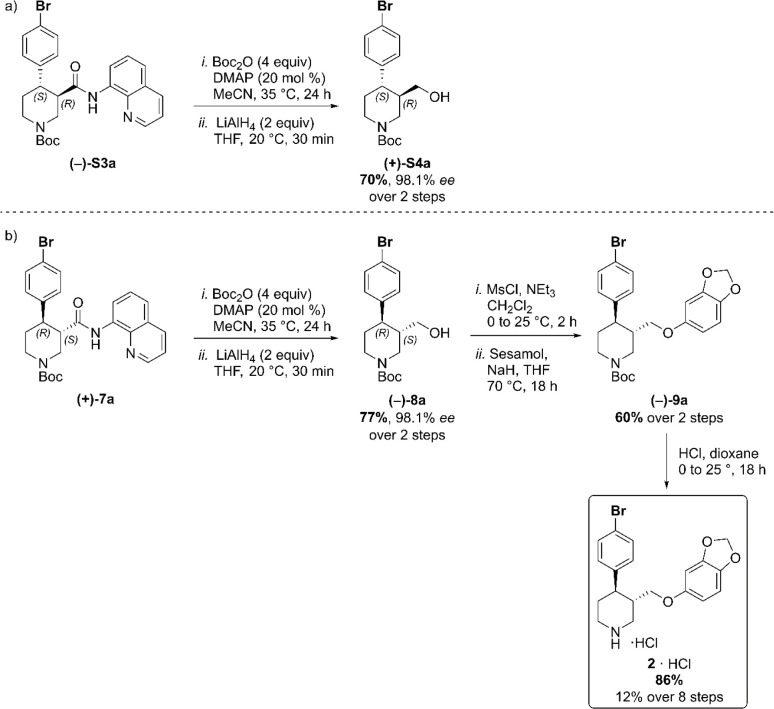
Appendix 1—scheme 2. Reductive aminoquinoline removal and final steps in the synthesis of Br-(–)-paroxetine 2.
(a) AQ removal on enantiomerically pure trans-piperidine (–)-S3a (0.2 mmol, one equiv). (b) AQ removal on enantiomerically pure trans-piperidine (+)−7a (1.1 mmol, one equiv) and final steps in the synthesis of Br-(–)-paroxetine 2. Full Synthetic Route to Racemic and Enantioenriched I-Piperidine Derivatives (±)-S2b, (±)-S3b, (+)−6b, (+)−7b, (–)-S3b, (–)−8b, (+)-S4b, (–)−9b and I-(–)-paroxetine 3 Similarly to the Br-analogue, C–H arylation with 1,4-diiodobenzene was performed on both racemic ((±)-S1) and enantioenriched ((–)−5) piperidine amide substrates (Scheme 3). The reaction proceeded well on both substrates affording racemic cis- and trans-arylated products (±)-S2b and (±)-S3b in 35% and 19% yield, and enantioenriched cis- and trans-derivatives (+)−6b and (–)-S3b in 35% and 20% yield respectively.