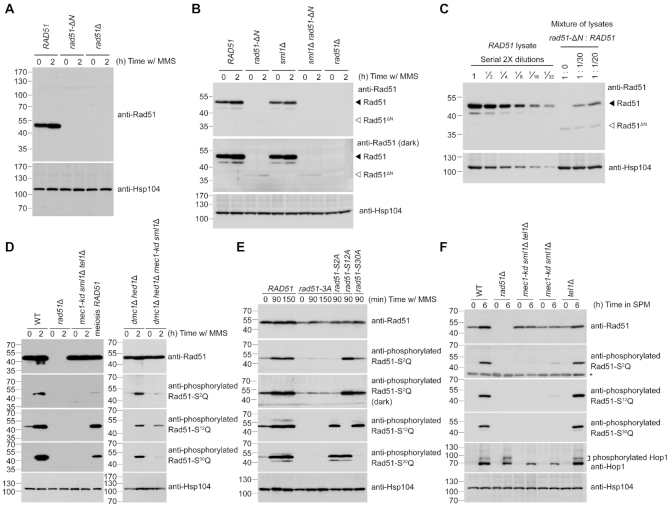
Figure 3.
Reduction of steady-state protein levels of Rad51-ΔN and the Mec1ATR- and Tel1ATM-dependent phosphorylation of Rad51-NTD. Total cell lysates were prepared from mitotic cells under MMS treatment (A-E), or from meiotic cells at indicated time-points after being transferred into sporulation media (SPM) (F), and then visualized by immunoblotting with the corresponding antisera. The asterisk indicates non-specific bands. Hsp104 was used as a loading control. Size in kilodaltons of standard protein markers is labeled to the left of the blots. (A) Absence of the NTD from Rad51-ΔN protein was confirmed by immunoblotting using goat anti-Rad51 antibody (yN-19) purchased from Santa Cruz Biotechnology (CA, USA). This antibody (sc-8936) was generated using a peptide mapping at the N-terminus of yeast Rad51. (B, C) Immunoblotting using guinea pig anti-Rad51 antisera that recognize the whole Rad51 protein (43), as depicted in Supplementary Figure S2A. The predicted molecular weight of Rad51-ΔN is 36,270 daltons, marked by white arrowheads to the right of the blots. Black arrowheads represent wild-type Rad51 at 42,944 daltons. (C) Total cell lysates from wild-type (WT) mitotic cells under MMS treatment were diluted with those of rad51-ΔN or empty sample buffer at indicated titers to estimate the relative steady-state protein levels of WT Rad51 and Rad51-ΔN. (E) Demonstration of the specificity of anti-phosphorylated Rad51-S2Q, Rad51-S12Q and Rad51-S30Q antisera. The darker exposure (third panel from top) illustrates that the phosphorylation-defective mutant proteins (Rad51-3A and Rad51-S2A) were slightly recognized by anti-phosphorylated Rad51-S2Q antisera.