Fig. 9.
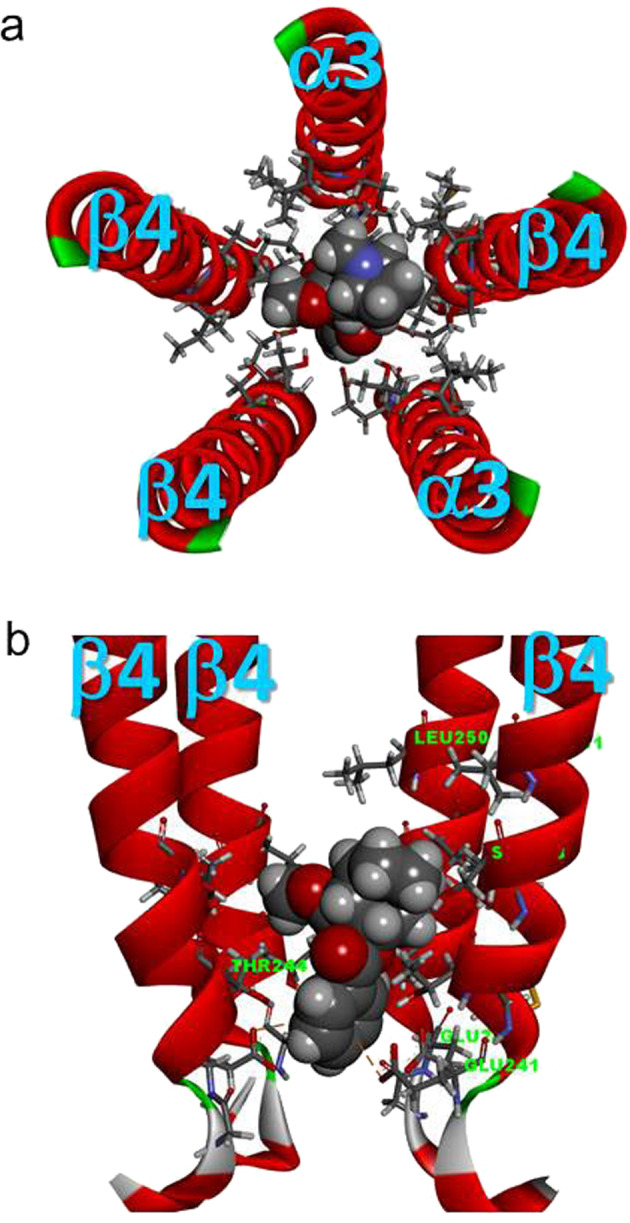
Cocaine molecule docked to the channel domain of the homology model of the human α3β4 nAChR with the subunit stoichiometry of (α3)2(β4)3. a Top view (from the extracellular side) of the channel-lining domain (with only M2 domains for clarity). The docked cocaine is located in the center of the pore and has a potential hydrogen bond with α3Ser247 (Chain A). b Side view of the channel-lining domain, with one α3 subunit (Chain D) removed from the front for clarity. The docked cocaine was located immediately above the selectivity filter (β4Glu241) up to the level at the 6′ position (α3Ser247, β4Ser248) of the second transmembrane domain (M2) with two potential π-anion interactions with two β4Glu241 residues of Chains C and D. The docked cocaine molecule was below the putative channel gate at the 9′ position (α3Leu250, β4Leu251)
