FIG. 1.
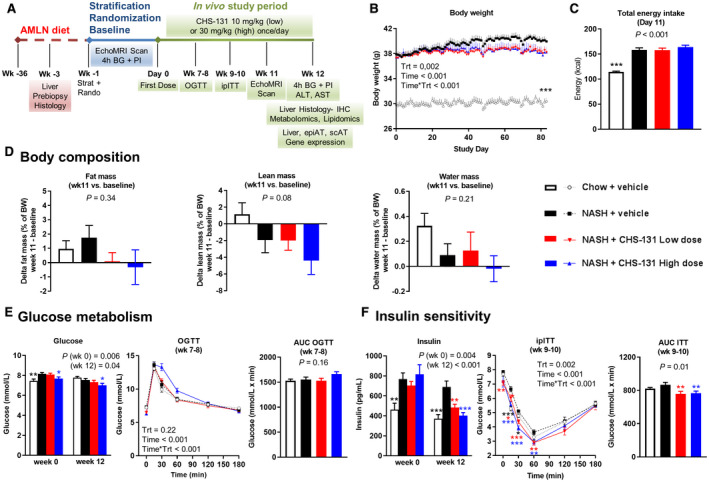
CHS‐131 improves insulin sensitivity without affecting body weight, body composition, energy intake, and glucose levels. (A) Schematic representation of the study design. (B) Body weight during the 12 weeks of treatment. Time is shown in minutes; Time*Trt indicates the interaction of parameters. (C) Total energy intake after 11 days of treatment. (D) Changes in body composition as percentage of body weight from baseline (start of treatment) up to week 11. (E) Glucose levels after 4 hours of fasting at start (week 0) and end (week 12) of treatment and during an OGTT at weeks 7‐8 (AUC of OGTT is also shown). (F) Insulin levels after 4 hours of fasting at start (week 0) and end (week 12) of treatment and during an ipITT at weeks 9‐10 (AUC of ipITT is also shown). One‐way ANOVA was performed for all single time‐point parameters (including body weight, which was analyzed only for the last study day) and two‐way ANOVA for parameters with multiple time points (OGTT, ipITT). For P < 0.05 (one‐way ANOVA) and for P < 0.05 for Trt (in two‐way ANOVA), one‐tailed *P < 0.05, **P < 0.01, ***P < 0.001, respectively, for post‐hoc LSD test for chow+vehicle (black stars) or NASH‐CHS‐131 low dose (red stars) or NASH‐CHS‐131 high dose (blue stars) compared to NASH+vehicle. Data show means ± SEMs. Abbreviations: AUC, area under the curve; BG, blood glucose; BW, body weight; PI, plasma insulin; min, minutes; Rando, randomized; Strat, stratification; Trt, treatment.
