Figure 1.
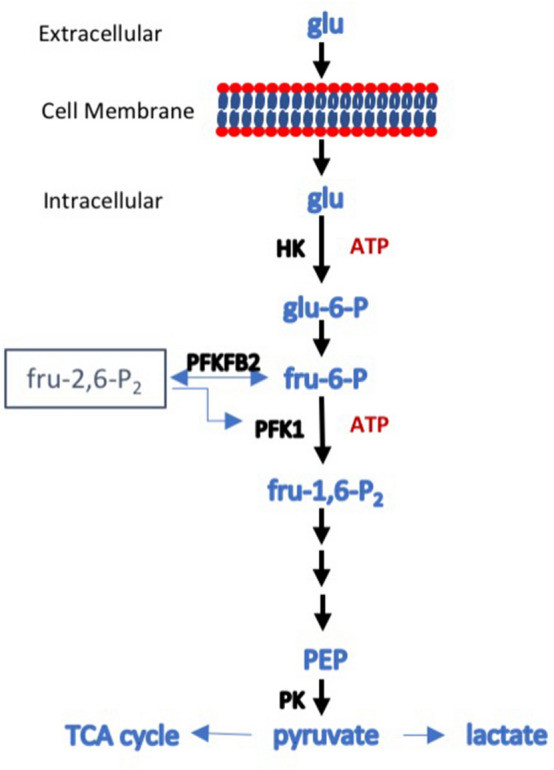
Schematic diagram of glycolysis. Glucose enters the cell via one of the glucose transporters and is phosphorylated, in an ATP-consuming step catalyzed in most cells by hexokinase (HK), to glucose-6-phosphate that cannot exit the cell. An isomerase converts it to fructose-6-phosphate, which is then converted to fructose-1,6-bisphosphate in a second ATP-consuming step catalyzed by phosphofructokinase 1 (PFK1). PFK1 catalysis is the major rate-limiting step in glycolysis and the first committed step. The activity of PFK1 is regulated by a number of factors, but one of the most important is the level of fructose-2,6-bisphosphate. Synthesis and degradation of fru-2,6-P2 is under the control of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFKFB), a bifunctional enzyme that catalyses both the formation and degradation of fru-2,6-P2. PFKFB exists as four isoforms, the products of separate genes, each with a distinct activity and tissue distribution. PFKFB2 has been described as the kidney isoform, as it is well expressed. Phosphorylation of PFKFB2 on either Serine366 and Serine382 increases formation of fru-2,6-P2 and increases the enzymatic activity of PFK1, driving glycolysis forward. The fate of fru-1,6-P2 is shown in outline. Fru-1,6-P2 is unstable and is converted to two 3-carbon sugars that eventually end as two molecules of phosphoenolpyruvate (PEP). This is then converted to pyruvate by pyruvate kinase (PK), which can then be converted to lactate, usually under anaerobic conditions, or enter the tricarboxylic acid (TCA) cycle in mitochondria, generally when there is oxygen available. In the diagram, only three glycolytic enzymes (HK, PFK1, PK) are shown as these are the rate limiting steps and the most susceptible to regulation.
