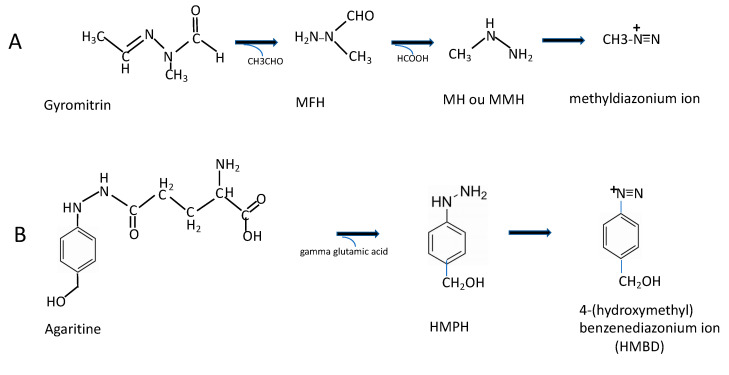
Figure 3.
Schematic view of degradation of two hydrazinic toxins, Gyromitrin and Agaritine, into instablediazonium ions (from which reactive radicals are generated): (A) Gyromitrin: (acetaldehyde methylformylhydrazone)→N-methyl-N-formylhydrazine (MFH)→monomethylhydrazine (=MH or MMH). (B) Agaritine: (β-N-[γ-L-(+)- glutamyl]-4-hydroxymethylphenylhydrazine) →4-(hydroxymethyl)phenylhydrazine (HMPH)→ 4- (hydroxymethyl)benzenediazonium ion (HMBD).