Abstract
Peripheral blood of cancer patients “physiologically” presents cells and cellular components deriving from primary or metastatic sites, including circulating tumor cells (CTCs), circulating free DNA (cfDNA) and exosomes containing proteins, lipids and nucleic acids. The term circulating tumor DNA (ctDNA) indicates the part of cfDNA which derives from primary tumors and/or metastatic sites, carrying tumor-specific genetic or epigenetic alterations. Analysis of ctDNA has enormous potential applications in all stages of cancer management, including earlier diagnosis of cancer, identification of driver alterations, monitoring of treatment response and detection of resistance mechanisms. Thus, ctDNA has the potential to profoundly change current clinical practice, by moving from tissue to peripheral blood as a source of information. Herein, we review current literature regarding the potential role for ctDNA in biliary tract cancer (BTC) patients, with a particular focus on state-of-the-art techniques and future perspectives of this highly aggressive disease.
Keywords: Liquid biopsy, biliary tract cancer, cholangiocarcinoma, ctDNA, cfDNA, review
Biliary tract cancers (BTCs) include a heterogeneous group of malignancies usually classified in the following subgroups, according to anatomical location: intrahepatic cholangio-carcinoma (iCCA), extrahepatic cholangiocarcinoma (eCCA), gallbladder cancer (GBC) and ampulla of Vater cancer (AVC) (1-3). The term cholangiocarcinoma includes iCCA and eCCA, which in turn comprises perihilar cholangiocarcinoma (pCCA) and distal cholangiocarcinoma (dCCA) (4,5). Although the anatomical classification of BTC may be considered simplistic, it faithfully reflects the differentiation of BTC subgroups in terms of epidemiology, etiology, clinical presentation, molecular features and therapeutic approaches (6,7). BTC currently represents about 3% of all gastrointestinal malignancies and the second most common primary liver cancer (PLC), following hepatocellular carcinoma (HCC) (8,9). Even though BTC is considered an uncommon cancer in Western countries, its incidence is increasing, and perhaps is associated with the increasing incidence of iCCA and partly as a result of better disease recognition (10-12). An important geographical variation in BTC epidemiology has been historically observed, with higher incidence rates in geographical areas where liver fluke infestation (Opistorchis viverrini and Clonorchis sinensis) is more common such as Korea, Japan, China and Thailand (13,14). More specifically, Northeast Thailand presents the highest BTC rate worldwide, with an annual incidence of 95/100,000 inhabitants and representing more than 80% of all PLCs in this region (15,16). Other countries such as India and Chile depict high incidence of GBC, given the high prevalence of chronic hepatolithiasis (17,18). Apart from these risk factors, primary sclerosing cholangitis (PSC), cirrhosis, chronic hepatitis C and B infection, fatty liver disease and asbestos exposure have been associated with an increased risk of developing BTC (19-22).
Although surgery remains the mainstay of cure in early stages, the majority of BTC patients are diagnosed with advanced-stage disease, therefore precluding any surgical management (23,24). Cisplatin plus gemcitabine combination chemotherapy is considered the standard first-line treatment in advanced, unresectable BTC, following the results of the ABC-02 landmark trial (25). Despite ABC-02 trial representing a historical step forward in medical treatment for advanced BTC, the survival gain provided by first-line chemotherapy is modest since nearly all patients develop progressive disease following front-line treatment, with a median overall survival (OS) of less than a year (26). More recently, although outstanding advances in genomic sequencing have given hope to new treatment strategies, BTC patients still have a poor prognosis with short life expectancy (27-29).
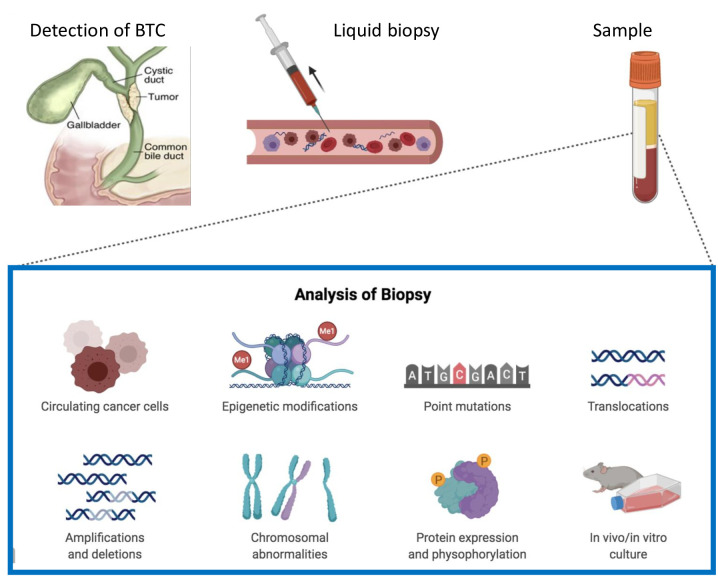
In the last decade, liquid biopsy has received growing attention because of its promising applications in patients with cancer (30,31). In fact, liquid biopsy, based on circulating free DNA (cfDNA), circulating tumor cells (CTCs), circulating cell-free RNA (ccfRNA) and circulating tumor DNA (ctDNA), represents a potential tool which could bring a new insight into cancer diagnosis and management (Figure 1) (32,33). More specifically, this new technology has the potential to reveal cancer-specific genetic and epigenetic features directly in the bloodstream (34,35); if the term cfDNA indicates DNA which is freely circulating but not necessarily of tumor origin, ctDNA represents a tumor-derived fragmented DNA which is released into the bloodstream (36,37). More specifically, the majority of cfDNA comes from normal cells; conversely, a small part of cfDNA directly comes from primary tumors, metastatic sites or CTCs, and it is called ctDNA (38-40). The possibility to detect biological, tumor-derived material circulating in body fluids may have remarkable applications in any phase of cancer management in terms of earlier diagnosis, detection of relapse, identification of therapeutic targets, monitoring of treatment response and tracking emergence of resistance (41-44).
Figure 1. Schematic figure of liquid biopsy as a source of information.
Herein, we review current literature regarding the potential clinical role of ctDNA in BTC management, with a particular focus on current state of art and possible future directions.
Current Limits in Diagnosis of BTC: Blood-based Markers, Imaging and Histology
Although multiple diagnostic methods are currently available, the diagnosis of BTC remains challenging (45,46). In clinical practice, CA19-9 and carcinoembryonic antigen (CEA) are the most frequently used blood-based tumor markers (47,48). However, CA 19-9 (with a cut-off >129 U/ml) represents the only recommended biomarker for clinical use, according to the ESMO guidelines for BTC (49); besides, overall sensitivity of CA19-9 remains controversial since high levels of CA19-9 may be encountered in several other malignancies, in benign cholestasis and after hepatic injuries (50). Lastly, various cut-off values have been proposed, usually between 100 U/ml and 200 U/ml (51).
Ultrasonography, computed tomography (CT) and magnetic resonance imaging (MRI) are important techniques for diagnosis and staging (52). At ultrasonography, iCCA appear as solid mass lesions while pCCA and dCCA are more difficult to identify using ultrasound (53); conversely, MRI is considered the modality of choice in BTC diagnosis, given the high contrast resolution and the ability to determine the vascular, biliary and parenchymal extension of the neoplasm (54).
Pathological confirmation of diagnosis is necessary before any non-surgical treatment and can be challenging in BTC, particularly in patients affected by PSC and biliary strictures (55). Decisions to undertake biopsies should follow a multidisciplinary discussion, especially in potentially resectable tumors (56). Endoscopic imaging and tissue sampling are useful but, unfortunately, biopsy samples are often inadequate for molecular profiling (57), and in addition, tissue sampling has reported high specificity but low sensitivity in diagnosis of malignant biliary strictures (58). Lastly, the highly desmoplastic nature of BTC limits the accuracy of cytological and pathological approaches (59).
In this scenario, it is urgent to develop new strategies in order to anticipate the diagnosis identifying BTC at an early, resectable stage, and obtain sufficient material with which to perform genomic analysis.
Genomic Profiling of BTC
Recent efforts in genomic sequencing and molecular subtyping have paved the way towards a new era in BTC management (60). In fact, the advances in the comprehension of BTC molecular landscape have recently provided new keys to identify prognostic and predictive biomarkers as well as mechanisms of resistance and pathogenesis (61). More specifically, almost 50% of BTCs are supposed to harbor at least one driver mutation, and to date, several targeted agents have shown promising results in recent clinical trials (62,63).
Firstly, Javle et al. suggested a correlation between genomic features and clinical outcomes, on the basis of data extracted from the FoundationOne platform (64). According to that study, KRAS was the most common aberration in eCCA (42% of cases), ERBB2 in GBC (16%) and IDH1 and FGFR in iCCA; moreover, FGFR mutations seemed to be associated with a good prognosis, according to the study. More recently, a multicenter study on 489 BTCs from 10 countries suggested the presence of 4 molecular clusters of BTC, on the basis of integrative clustering analysis of mutations, combined whole-genome, copy-number, gene expression and DNA methylation data (65). In this study, Cluster 1 mainly included fluke-positive malignancies with ERBB2 amplification, TP53 and ARID1A alterations; conversely, Cluster 4 identified fluke-negative iCCA with FGFR aberrations and CpG shore hypermethylation. Moreover, better OS was observed in Cluster 4, thus supporting previous findings from Javle et al. regarding the role of FGFR aberrations.
As stated above, these aberrations and molecular features represent potential therapeutic targets in specific anatomic subtypes. The recent prospective MOSCATO-1 trial analyzed 1,035 tumor samples and matched, on the basis of genetic aberrations, 199 patients to specific targeted therapies (66). Among them, 18 patients were affected by previously treated, advanced BTC; interestingly, in BTC patients receiving targeted therapies ORR was 33% and PFS and OS were 5.2 months and 17 months, respectively.
A plethora of previous studies on BTC have grouped together patients with different anatomical and molecular subtypes, something which represents the “original sin” of several clinical trials which do not do justice to the marked inter- and intra-tumoral heterogeneity of BTC (67-69). The modest survival benefit observed with current treatment options emphasizes the need for new affective agents and tailor-made trials based on genetic profile and histological features characterizing BTC (70). The emergence of targeted treatments in BTC is challenging previous treatment paradigms, especially for iCCA for whom targeting FGFR fusions and IDH1/IDH2 mutations is becoming part of current clinical practice (71-73).
Between Two Worlds: ctDNA Assay and Tissue-based Assay
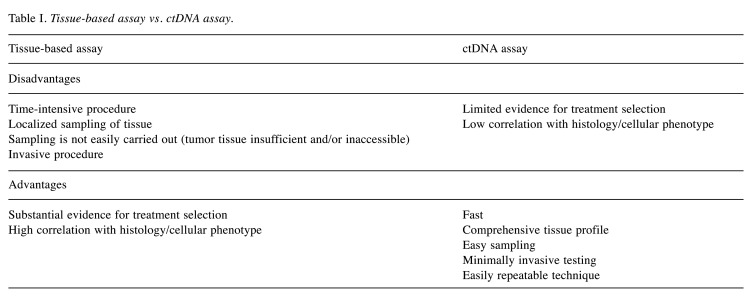
Tumor biopsies are the gold standard for cancer diagnosis and the primary tool for molecular testing, guiding treatment selection (74). Nevertheless, sampling tissue is an invasive and often anatomically difficult method; moreover, conventional tissue biopsies are not always feasible, they frequently need to be repeated and it is not easy to obtain sufficient material of proper quality for cancer genome profiling (75).
Conversely, the analysis of ctDNA has the potential to overcome the abovementioned limitations, by capturing the outstanding spatial and temporal tumor heterogeneity and expanding the opportunity for real-time monitoring (76). Thus, liquid biopsy is emerging as a promising, attractive molecular diagnostic tool with minimal invasiveness (77). Compared to classic tissue biopsies, the analysis of ctDNA is quick, simple and presents minimal procedural risk (Table I), considering that blood, saliva or urine are easier to access than tissue biopsy (78). In fact, although liquid biopsy is commonly referred to peripheral blood analysis, this term includes the collection and analysis of cancer-derived material from other bodily fluids such as saliva, bile, urine, stool, cerebrospinal fluid, ascites and pleural fluid (79). Overall, liquid biopsy is the natural “partner” of tailor-made, personalized oncology approach, having the potential to capture tumor spatio-temporal heterogeneity and providing a more “holistic” view of tumor (80-82).
Table I. Tissue-based assay vs. ctDNA assay.
Limitations of cfDNA/ctDNA analysis include lack of spatial specificity for anatomically critical and clinically relevant lesions, low shedding of ctDNA by certain malignancies and the lack of prospective validation for clinical practice for a majority of cancers (83,84). Moreover, currently available ctDNA assays are not able to detect a number of genes compared to tissue-based panels, a critical issue which modern technologies are trying to face (85-87).
Clinical Applications of ctDNA/cfDNA Analysis
In 1948, Mandel and Métais were the first to identify fragmented DNA in the non-cellular component of the blood, which was called cfDNA (88). Twenty-nine years after the first identification of cfDNA, Leon et al. observed increased levels of cfDNA in cancer patients compared to healthy controls (89). Since then, an accumulating body of literature has investigated CTCs, ctDNA and cfDNA as novel biomarkers, with the aim to facilitate early detection of malignancies and improve the prognosis of cancer patients (90-92). On the basis of current knowledge, the mechanisms of apoptosis and necrosis have been identified as important contributors to cfDNA release into the bloodstream (93). In physiological conditions, cfDNA derived from cells is found in plasma at low concentrations which may be influenced by several stressing situations (e.g. physical exercise, surgery, inflammation, etc.) (94). As previously stated, the proportion of cfDNA which is specifically released from tumor cells is currently called ctDNA, who in turn may represent from 0.1% to 90% of overall cfDNA (95). The applications of cfDNA/ctDNA can be schematically summarized by five categories (Table II): diagnosis, detection of tumor burden, prognosis, selection of treatment and monitoring for relapse/treatment efficacy.
Table II. Highlights of current and future applications of ctDNA in cancer management.
With regard to diagnosis, early detection methods are under active investigation (96). In particular, the diagnosis of cancer at an early stage remains a challenge in several malignancies, given the frequent “silent” clinical character of early-stage disease and, in many cases, even of advanced cancer (97). Therefore, identifying early-stage malignancies would mean better chance of cure making cfDNA analysis an extremely attractive tool (98). Unfortunately, this approach would need an extremely sensitive method in order to detect minimal amounts of cfDNA released into the bloodstream and to date, no technology currently exists to reach this goal (99).
In the current era of precision cancer therapies, the choice of treatment is often based on tumor molecular profile and the clinical benefit of tailor-made agents is limited by the emergence of acquired resistance (100). In this landscape, ctDNA has the potential to assess molecular profile with a quick and minimally invasive procedure such as a simple blood draw (101). Despite early studies detected low concordance between tumor and plasma samples, recent and larger studies have suggested concordance rates from 80% to 90% between the two samples, particularly in key driver genes (102,103). Nevertheless, a proportion of patients affected by metastatic disease (estimated at about 10-15%) may not present sufficient cfDNA/ctDNA levels to permit mutational profiling from plasma, a key element to consider when interpreting the results of cfDNA/ctDNA analysis (104).
During medical treatment, liquid biopsy may detect emergent genetic alterations driving acquired therapeutic resistance (105); thus, serial liquid biopsies may be useful tools to identify resistant mutations and to change treatment in real time, avoiding invasive tumor biopsies (106). Therefore, liquid biopsy has rapidly emerged as an extremely promising technology, due to the ability to capture tumor molecular heterogeneity and the clonal outgrowth of resistant subclones (107). Interestingly, the main and earliest example of the application of cfDNA/ctDNA testing for the management of therapeutic resistance is epidermal growth factor receptor (EGFR)-mutated non-small cell lung cancer (NSCLC) (108,109). In this setting, cfDNA analysis may detect the emergence of EGFR T790M mutation during EGFR inhibitor therapy – with a high level of concordance observed between the results of tissue testing and liquid biopsy – and also the coexistence of other resistance mechanisms, such as MET amplification (110). Other possible applications of cfDNA/ctDNA include the identification of prognostic and predictive biomarkers, detection of postsurgical residual disease, tracking of therapeutic response and the detection of recurrence (111,112). Despite the fact that liquid biopsy may pave the way for a revolution in medical oncology, a careful understanding of limitations and advantages of this approach is mandatory to properly interpret the analysis and to correctly guide clinical decision making.
The Use of ctDNA in BTC
Although liquid biopsy may present an attractive diagnostic tool in early-stage BTC, very few data are currently available and the advances in the field have been hampered by technical challenges primarily due to the frequently low levels of ctDNA in patients with localized disease (113). As stated above, the difficulty in obtaining sufficient cytologic material to confirm the diagnosis and to perform genomic analysis is particularly challenging in BTC, whose poor prognosis is in part due to late diagnosis (114). Thus, ctDNA could play a particularly important role in BTC patients, since biopsy samples are often inadequate for molecular profiling, especially in eCCA and GBC (115).
In a prospective analysis of 26 pancreatobiliary malignancies, Zill et al. reported high concordance between mutations detected in tumor biopsies and cfDNA (116). This study included 8 patients with BTC and 18 with pancreatic cancer; cfDNA identified the 90.3% of mutations detected in tissue biopsies.
A study by Kumari et al. recently assessed the role of cfDNA in the diagnosis of GBC (117). Serum was collected from 34 GBC patients and 39 sex- and age-matched controls, 22 of which with cholecystitis and 17 patients without comorbidities. In this study, which represented the first to evaluate serum cfDNA in GBC, the authors used real-time PCR assay to quantify amount of cfDNA, comparing the three cohorts of patients (117). Interestingly, cfDNA was found to be significantly lower in cholecystitis controls and healthy subjects compared to the GBC group. Moreover, cfDNA was significantly associated with jaundice, metastatic lymph nodes and stage, according to TNM system (117). Thus, cfDNA quantitative analysis could play an important role in distinguishing inflammatory disorders and GBC and may serve as novel, noninvasive marker for GBC diagnosis.
In another study on 69 cholangiocarcinoma patients (94% with pCCA) and 95 healthy sex- and age-matched controls, cfDNA analysis identified a panel of four genes (HOXA1, PRKCB, CYP26C1, and PTGDR) which had differentially methylated regions (DMRs) in CCA patients (118). The panel showed a specificity of 93% and a sensitivity of 83% in the detection of cholangiocarcinoma; interestingly, the DMR ctDNA panel detected 32 (80%) of the 40 CCAs which were deemed eligible for surgical resection or transplantation and 15 (60%). Overall, the sensitivity of cfDNA/ctDNA mutations for early stage BTC is currently unknown.
As previously stated, sequencing of tissue samples may be limited by low tumoral content, thus liquid biopsy is being harnessed for genomic profiling of BTC (119). In a study by Andersen and Jakobsen, the authors proposed a multiplex digital PCR method of screening for 31 mutations in KRAS, NRAS, BRAF and PIK3CA genes in patient plasma (120). Interestingly, the assay was firstly confirmed in pooled normal serum and positive controls; therefore, the assay was conducted on serum of six wild-type patients for the assayed mutations and five BTC patients with proven tumor mutations. Mutations found in the tumor were in parallel found in the plasma of all the “mutated” patients and, at the same time, there was a perfect agreement in wild-type status between tumor and plasma (120).
In another study, Mody et al. performed a ctDNA analysis on 138 samples of BTC patients, finding at least one genomic alteration in 89% of cases (121). Interestingly, the majority of cases included in this study were iCCAs, something which represents the main limitation of this study since iCCAs are the BTC subgroup for which liver biopsies and tissue sampling are easier. Although the most frequently detected alterations were TP53, KRAS and FGFR2, the proper and parallel concordance between ctDNA and tissue-based alterations has yet to be assessed in larger cohorts of patients (121).
Another role for ctDNA/cfDNA is represented by monitoring response to chemotherapy and targeted therapy, thus tracking emergence of resistance (122,123). In a German study, ctDNA and tumor tissue samples were collected from 24 BTC patients before and during chemotherapy; the two samples were subjected to deep sequencing of 15 frequently mutated genes in BTC, including TP53, ARID1A, KRAS, IDH1, BAP1, PBRM1, SMAD4, PIK3CA, FBXW7, CDKN2A, ERBB2, NRAS, IDH2, BRAF and BLC2 (124). Interestingly, ctDNA in blood compared to tissue had a concordance of 74% in all patients and 92% in the iCCA cohort; moreover, 63% of chemotherapy-naïve patients had their mutational profile changed during treatment. Lastly, ctDNA variant allele frequency (VAF) showed a strict correlation with progression-free survival (PFS) and tumor load.
As previously stated, FGFR2 genomic alterations are the most frequently observed aberrations in iCCA, with a prevalence ranging from 13-45% and a mutual exclusivity with KRAS/BRAF mutation (125,126). In recent years, the role of FGFR-targeted therapies has been tested in a number of clinical trials and various agents have been evaluated or are currently under investigation including multitarget tyrosine kinase inhibitors as well as specific anti-FGFR2 antibodies including BGJ39 (127). Goyal et al. recently analyzed cfDNA collected by serial sampling in 4 patients enrolled in a Phase II trial assessing the role of BGJ39 (128). Among the 4 patients, 3 experienced significant tumor regression followed by short interval disease progression. Serial analysis of cfDNA at enrollment and after progression showed the presence of the V564F acquired mutation at the time of progression and, in 2 patients, multiple point mutations in the FGFR portion of the fusion genes (128). Moreover, a high concordance was observed between tissue and plasma measurements, since tumor biopsy of the post-progression lesions and postmortem analysis agreed with cfDNA analysis, identifying marked intratumor heterogeneity and de novo point mutations conferring resistance to the FGFR inhibitor (128). Although based on a small subgroup of patients, the study highlighted the potential advantages of cfDNA in BTC targeted therapy, where real-time detection of resistance mutations and monitoring of clonal evolution may provide extremely useful information to guide the selection of treatment.
Lastly, the option to use the bile as source for DNA sequencing in BTC has been recently investigated and deserves to be mentioned, since bile is another component of liquid biopsy. A recent study by Shen et al. from 10 BTC patients (including 4 cases of GBC) suggested that bile cfDNA could consist of long fragments, with a high correspondence between molecular features detected in bile and tissue sampling (129). Studies on larger cohorts of patients are needed to confirm the above results and to further assess the role of bile as source of cfDNA.
Conclusion
The applications of ctDNA/cfDNA on tumor detection, characterization and genetic assessment have the potential to pave the way towards a new era in cancer management. Although few data are currently available regarding ctDNA analysis in BTC, this cost-effective, fast and non-invasive test may contribute to the implementation of precision medicine and improve clinical outcomes in a highly aggressive and increasingly frequent disease.
Conflicts of Interest
No potential conflict of interest was reported by the Authors.
Authors’ Contributions
AR, ADR: Made substantial contributions to the conception of the study and drafted the article; ST, GB: critically revised the article and gave final approval of the version to be published. All Authors critically revised the article, approved the final version to be published, and agree to be accountable for all aspects of the work.
References
- 1.Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int. 2019;39(Suppl 1):19–31. doi: 10.1111/liv.14095. [DOI] [PubMed] [Google Scholar]
- 2.Adeva J, Sangro B, Salati M, Edeline J, La Casta A, Bittoni A, Berardi R, Bruix J, Valle JW. Medical treatment for cholangiocarcinoma. Liver Int. 2019;39(Suppl 1):123–142. doi: 10.1111/liv.14100. [DOI] [PubMed] [Google Scholar]
- 3.Rizzo A, Frega G, Ricci AD, Palloni A, Abbati F, DE Lorenzo S, Deserti M, Tavolari S, Brandi G. Anti-EGFR monoclonal antibodies in advanced biliary tract cancer: A systematic review and meta-analysis. In Vivo. 2020;34(2):479–488. doi: 10.21873/invivo.11798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145(6):1215–1229. doi: 10.1053/j.gastro.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383(9935):2168–2179. doi: 10.1016/S0140-6736(13)61903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma – evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15(2):95–111. doi: 10.1038/nrclinonc.2017.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer. 2002;2:10. doi: 10.1186/1471-2407-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fornaro L, Cereda S, Aprile G, Di Girolamo S, Santini D, Silvestris N, Lonardi S, Leone F, Milella M, Vivaldi C, Belli C, Bergamo F, Lutrino SE, Filippi R, Russano M, Vaccaro V, Brunetti AE, Rotella V, Falcone A, Barbera MA, Corbelli J, Fasola G, Aglietta M, Zagonel V, Reni M, Vasile E, Brandi G. Multivariate prognostic factors analysis for second-line chemotherapy in advanced biliary tract cancer. Br J Cancer. 2014;110(9):2165–2169. doi: 10.1038/bjc.2014.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Lorenzo S, Tovoli F, Barbera MA, Garuti F, Palloni A, Frega G, Garajova I, Rizzo A, Trevisani F, Brandi G. Metronomic capecitabine vs. best supportive care in Child-Pugh B hepatocellular carcinoma: a proof of concept. Sci Rep. 2018;8:9997. doi: 10.1038/s41598-018-28337-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jepsen P, Vilstrup H, Tarone RE, Friis S, Sorensen HT. Incidence rates of intra- and extrahepatic cholangiocarcinomas in Denmark from 1978 through 2002. J Natl Cancer Inst. 2007;99(11):895–897. doi: 10.1093/jnci/djk201. [DOI] [PubMed] [Google Scholar]
- 11.Brandi G, Farioli A, Astolfi A, Biasco G, Tavolari S. Genetic heterogeneity in cholangiocarcinoma: a major challenge for targeted therapies. Oncotarget. 2015;6(17):14744–14753. doi: 10.18632/oncotarget.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaib YH, Davila JA, McGlynn K, El-Serag HB. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase. J Hepatol. 2004;40(3):472–477. doi: 10.1016/j.jhep.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 13.Saha SK, Zhu AX, Fuchs CS, Brooks GA. Forty-year trends in cholangiocarcinoma incidence in the US: intrahepatic disease on the rise. Oncologist. 2016;21:594–599. doi: 10.1634/theoncologist.2015-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smittenaar CR, Petersen KA, Stewart K, Moitt N. Cancer incidence and mortality projections in the UK until 2035. Br J Cancer. 2016;115(9):1147–1155. doi: 10.1038/bjc.2016.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palmer WC, Patel T. Are common factors involved in the pathogenesis of primary liver cancers? A meta-analysis of risk factors for intrahepatic cholangiocarcinoma. J Hepatol. 2012;57(1):69–76. doi: 10.1016/j.jep.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan SA, Taylor-Robinson SD, Toledano MB, Beck A, Elliott P, Thomas HC. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol. 2002;37:806–813. doi: 10.1016/s0168-8278(02)00297-0. [DOI] [PubMed] [Google Scholar]
- 17.Lin CC, Lin PY, Chen YL. Comparison of concomitant and subsequent cholangiocarcinomas associated with hepatolithiasis: clinical implications. World J Gastroenterol. 2013;19(3):375–380. doi: 10.3748/wjg.v19.i3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tyson GL, El-Serag HB. Risk factors for cholangio-carcinoma. Hepatology. 2011;54(1):173–184. doi: 10.1002/hep.24351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang H, Zhu B, Zhang H, Liang J, Zeng W. HBV infection status and the risk of cholangiocarcinoma in Asia: a meta-analysis. Biomed Res Int. 2016;2016:3417976. doi: 10.1155/2016/3417976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brandi G, Tavolari S. Asbestos and intrahepatic cholangiocarcinoma. Cells. 2020;9(2) doi: 10.3390/cells9020421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sastry AV, Abbadessa B, Wayne MG, Steele JG, Cooperman AM. What is the incidence of biliary carcinoma in choledochal cysts, when do they develop, and how should it affect management. World J Surg. 2015;39(2):487–492. doi: 10.1007/s00268-014-2831-5. [DOI] [PubMed] [Google Scholar]
- 22.Jang MH, Lee YJ, Kim H. Intrahepatic cholangiocarcinoma arising in Caroli's disease. Clin Mol Hepatol. 2014;20(4):402–405. doi: 10.3350/cmh.2014.20.4.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farioli A, Straif K, Brandi G, Curti S, Kjaerheim K, Martinsen JI, Sparen P, Tryggvadottir L, Weiderpass E, Biasco G, Violante FS, Mattioli S, Pukkala E. Occupational exposure to asbestos and risk of cholangiocarcinoma: a population-based case-control study in four Nordic countries. Occup Environ Med. 2018;75(3):191–198. doi: 10.1136/oemed-2017-104603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forner A, Vidili G, Rengo M, Bujanda L, Ponz-Sarvisé M, Lamarca A. Clinical presentation, diagnosis and staging of cholangiocarcinoma. Liver Int. 2019;39(Suppl 1):98–107. doi: 10.1111/liv.14086. [DOI] [PubMed] [Google Scholar]
- 25.Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J, ABC-02 Trial Investigators Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 26.Brandi G, Rizzo A, Dall’Olio FG, Felicani C, Ercolani G, Cescon M, Frega G, Tavolari S, Palloni A, De Lorenzo S, Abbati F, Mollica V, Ricci AD, Serra C. Percutaneous radiofrequency ablation in intrahepatic cholangiocarcinoma: a retrospective single-center experience. Intl J Hyperthermia. 2020;37:479–485. doi: 10.1080/02656736.2020.1763484. [DOI] [PubMed] [Google Scholar]
- 27.Robertson S, Hyder O, Dodson R, Nayar SK, Poling J, Beierl K, Eshleman JR, Lin MT, Pawlik TM, Anders RA. The frequency of KRAS and BRAF mutations in intrahepatic cholangiocarcinomas and their correlation with clinical outcome. Hum Pathol. 2013;44(12):2768–2773. doi: 10.1016/j.humpath.2013.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Voss JS, Holtegaard LM, Kerr SE, Fritcher EG, Roberts LR, Gores GJ, Zhang J, Highsmith WE, Halling KC, Kipp BR. Molecular profiling of cholangiocarcinoma shows potential for targeted therapy treatment decisions. Hum Pathol. 2013;44(7):1216–1222. doi: 10.1016/j.humpath.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer. 2007;96:896–902. doi: 10.1038/sj.bjc.6603648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rizzo A, Mollica V, Ricci AD, Maggio I, Massucci M, Rojas Limpe FL, Fabio FD, Ardizzoni A. Third- and later-line treatment in advanced or metastatic gastric cancer: a systematic review and meta-analysis. Future Oncol. 2020;16(2):4409–4418. doi: 10.2217/fon-2019-0429. [DOI] [PubMed] [Google Scholar]
- 31.Mollica V, Di Nunno V, Santoni M, Cimadamore A, Scarpelli M, Lopez-Beltran A, Cheng L, Mariani C, Battelli N, Montironi R, Massari F. An evaluation of current prostate cancer diagnostic approaches with emphasis on liquid biopsies and prostate cancer. Expert Rev Mol Diagn. 2020;20(2):207–217. doi: 10.1080/14737159.2019.1684265. [DOI] [PubMed] [Google Scholar]
- 32.Santoni M, Massari F, Del Re M, Ciccarese C, Piva F, Principato G, Montironi R, Santini D, Danesi R, Tortora G, Cascinu S. Investigational therapies targeting signal transducer and activator of transcription 3 for the treatment of cancer. Expert Opin Investig Drugs. 2015;24(6):809–824. doi: 10.1517/13543784.2015.1020370. [DOI] [PubMed] [Google Scholar]
- 33.Maly V, Maly O, Kolostova K, Bobek V. Circulating tumor cells in diagnosis and treatment of lung cancer. In Vivo. 2019;33(4):1027–1037. doi: 10.21873/invivo.11571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kolostova K, Rzechonek A, Schützner J, Grill R, Lischke R, Hladik P, Simonek J, Bobek V. Circulating tumor cells as an auxiliary diagnostic tool in surgery. In Vivo. 2017;31(6):1197–1202. doi: 10.21873/invivo.11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ricci AD, Rizzo A, Novelli M, Tavolari S, Palloni A, Tober N, Abbati F, Mollica V, DE Lorenzo S, Turchetti D, DI Marco M, Brandi G. Specific toxicity of maintenance olaparib versus placebo in advanced malignancies: a systematic review and meta-analysis. Anticancer Res. 2020;40(2):597–608. doi: 10.21873/anticanres.13989. [DOI] [PubMed] [Google Scholar]
- 36.Nygaard AD, Holdgaard PC, Spindler KL, Pallisgaard N, Jakobsen A. The correlation between cell-free DNA and tumour burden was estimated by PET/CT in patients with advanced NSCLC. Br J Cancer. 2014;110:363–368. doi: 10.1038/bjc.2013.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dawson S-J, Tsui JW, Murtaza M, Biggs H, Rueda OM, Chin SF, Dunning MJ, Gale D, Forshew T, Mahler-Arauho B, Rajan S, Humphray S, Becq J, Halsall D, Wallis M, Bentley D, Caldas C, Rosenfeld D. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med. 2013;368:1199–1209. doi: 10.1056/NEJMoa1213261. [DOI] [PubMed] [Google Scholar]
- 38.Cimadamore A, Massari F, Santoni M, Mollica V, Di Nunno V, Cheng L, Lopez-Beltran A, Scarpelli M, Montironi R, Moch H. Molecular characterization and diagnostic criteria of renal cell carcinoma with emphasis on liquid biopsies. Expert Rev Mol Diagn. 2020;20(2):141–150. doi: 10.1080/14737159.2019.1665510. [DOI] [PubMed] [Google Scholar]
- 39.Lee SY, Chae DK, An J, Yoo S, Jung S, Chae CH, Bhak J, Kim BC, Cho DH. Combinatory analysis of cell-free and circulating tumor cell DNAs provides more variants for cancer treatment. Anticancer Res. 2019;39(12):6595–6602. doi: 10.21873/anticanres.13875. [DOI] [PubMed] [Google Scholar]
- 40.Buono G, Gerratana L, Bulfoni M, Provinciali N, Basile D, Giuliano M, Corvaja C, Arpino G, Del Mastro L, De Placido S, De Laurentiis M, Cristofanilli M, Puglisi F. Circulating tumor DNA analysis in breast cancer: Is it ready for prime-time. Cancer Treat Rev. 2019;73:73–83. doi: 10.1016/j.ctrv.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka R, Kimura K, Eguchi S, Tauchi J, Shibutani M, Shinkawa H, Ohira G, Yamazoe S, Tanaka S, Amano R, Tanaka H. Preoperative neutrophil-to-lymphocyte ratio predicts tumor-infiltrating CD8+ T cells in biliary tract cancer. Anticancer Res. 2020;40(5):2881–2887. doi: 10.21873/anticanres.14264. [DOI] [PubMed] [Google Scholar]
- 42.Rizzo A, Pantaleo MA, Saponara S, Nannini M. Current status of the adjuvant therapy in uterine sarcoma: A literature review. World J Clin Cases. 2019;7(14):1753–1763. doi: 10.12998/wjcc.v7.i14.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Modena A, Ciccarese C, Iacovelli R, Brunelli M, Montironi R, Fiorentino M, Tortora G, Massari F. Immune checkpoint inhibitors and prostate cancer: a new frontier. Oncol Rev. 2016;10(1):293. doi: 10.4081/oncol.2016.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Komiya K, Nakashima C, Nakamura T, Hirakawa H, Abe T, Ogusu S, Takahashi K, Takeda Y, Egashira Y, Kimura S, Sueoka-Aragane N. Current status and problems of T790M detection, a molecular biomarker of acquired resistance to EGFR tyrosine kinase inhibitors, with liquid biopsy and re-biopsy. Anticancer Res. 2018;38(6):3559–3566. doi: 10.21873/anticanres.12628. [DOI] [PubMed] [Google Scholar]
- 45.Brandi G, Venturi M, Pantaleo MA, Ercolani G, GICO Cholangiocarcinoma: Current opinion on clinical practice diagnostic and therapeutic algorithms: A review of the literature and a long-standing experience of a referral center. Dig Liver Dis. 2016;48(3):231–241. doi: 10.1016/j.dld.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 46.Joo I, Lee JM, Yoon JH. Imaging diagnosis of intrahepatic and perihilar cholangiocarcinoma: recent advances and challenges. Radiology. 2018;288(1):7–13. doi: 10.1148/radiol.2018171187. [DOI] [PubMed] [Google Scholar]
- 47.Xu MM, Sethi A. Diagnosing biliary malignancy. Gastrointest Endosc Clin N Am. 2015;25(4):677–690. doi: 10.1016/j.giec.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 48.Patel AH, Harnois DM, Klee GG, LaRusso NF, Gores GJ. The utility of CA 19-9 in the diagnoses of cholangiocarcinoma in patients without primary sclerosing cholangitis. Am J Gastroenterol. 2000;95(1):204–207. doi: 10.1111/j.1572-0241.2000.01685.x. [DOI] [PubMed] [Google Scholar]
- 49.Valle JW, Borbath I, Khan SA, Huguet F, Gruenberger T, Arnold D, ESMO Guidelines Committee Biliary cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v28–v37. doi: 10.1093/annonc/mdw324. [DOI] [PubMed] [Google Scholar]
- 50.Rahnemai-Azar AA, Weisbrod A, Dillhoff M, Schmidt C, Pawlik TM. Intrahepatic cholangiocarcinoma: Molecular markers for diagnosis and prognosis. Surg Oncol. 2017;26(2):125–137. doi: 10.1016/j.suronc.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 51.Levy C, Lymp J, Angulo P, Gores GJ, Larusso N, Lindor KD. The value of serum CA 19-19 in predicting cholangiocarcinomas in patients with primary sclerosing cholangitis. Dig Dis Sci. 2005;50:1734–1740. doi: 10.1007/s10620-005-2928-8. [DOI] [PubMed] [Google Scholar]
- 52.Iavarone M, Piscaglia F, Vavassori S, Galassi M, Sangiovanni A, Venerandi L, Forzenigo LV, Golfieri R, Bolondi L, Colombo M. Contrast enhanced CT-scan to diagnose intrahepatic cholangiocarcinoma in patients with cirrhosis. J Hepatol. 2013;58:1188–1193. doi: 10.1016/j.jhep.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 53.Nehls O, Gregor M, Klump B. Serum and bile markers for cholangiocarcinoma. Semin Liver Dis. 2004;24:139–154. doi: 10.1055/s-2004-828891. [DOI] [PubMed] [Google Scholar]
- 54.Oliveira IS, Kilcoyne A, Everett JM, Mino-Kenudson M, Harisinghani MG, Ganesan K. Cholangiocarcinoma: classification, diagnosis, staging, imaging features, and management. Abdom Radiol. 2017;42(6):1637–1649. doi: 10.1007/s00261-017-1094-7. [DOI] [PubMed] [Google Scholar]
- 55.Cravo M. Is CA 19-9 of any help in the management of cholangiocarcinoma. GE Port J Gastroenterol. 2017;24(3):108–109. doi: 10.1159/000457910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamashita S, Passot G, Aloia TA, Chun YS, Javle M, Lee JE, Vauthey JN, Conrad C. Prognostic value of carbohydrate antigen 19-9 in patients undergoing resection of biliary tract cancer. Br J Surg. 2017;104(3):267–277. doi: 10.1002/bjs.10415. [DOI] [PubMed] [Google Scholar]
- 57.Squadroni M, Tondulli L, Gatta G, Mosconi S, Beretta G, Labianca R. Cholangiocarcinoma. Crit Rev Oncol Hematol. 2017;116:11–31. doi: 10.1016/j.critrevonc.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 58.Taghavi SA, Eshraghian A, Niknam R, Sivandzadeh GR, Bagheri Lankarani K. Diagnosis of cholangiocarcinoma in primary sclerosing cholangitis. Expert Rev Gastroenterol Hepatol. 2018;12(6):575–584. doi: 10.1080/17474124.2018.1473761. [DOI] [PubMed] [Google Scholar]
- 59.Baiu I, Visser B. Gallbladder Cancer. JAMA. 2018;320(12):1294. doi: 10.1001/jama.2018.11815. [DOI] [PubMed] [Google Scholar]
- 60.Massard C, Michiels S, Ferté C, Le Deley MC, Lacroix L, Hollebecque A, Verlingue L, Ileana E, Rosellini S, Ammari S, Ngo-Camus M, Bahleda R, Gazzah A, Varga A, Postel-Vinay S, Loriot Y, Even C, Breuskin I, Auger N, Job B, De Baere T, Deschamps F, Vielh P, Scoazec JY, Lazar V, Richon C, Ribrag V, Deutsch E, Angevin E, Vassal G, Eggermont A, André F, Soria JC. High-throughput genomics and clinical outcome in hard-to-treat advanced cancers: results of the MOSCATO 01 trial. Cancer Discov. 2017;7(6):586–595. doi: 10.1158/2159-8290.CD-16-1396. [DOI] [PubMed] [Google Scholar]
- 61.Valle JW, Lamarca A, Goyal L, Barriuso J, Zhu AX. New horizons for precision medicine in biliary tract cancers. Cancer Disc. 2017;7(9):943–962. doi: 10.1158/2159-8290.CD-17-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lamarca A, Galdy S, Barriuso J, Moghadam S, Beckett E, Rogan J, Backen A, Billington C, McNamara MG, Hubner RA, Cramer A, Valle JW. The HER3 pathway as a potential target for inhibition in patients with biliary tract cancers. PLoS One. 2018;13:e0206007. doi: 10.1371/journal.pone.0206007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mazzaferro V, el-Rayes BF, Cotsoglou C, Harris WP, Damjanov N, Masi G, Rimassa L, Personeni N, Braiteh FS, Zagonel V, Papadopoulos KP, Hall T, Wang Y, Abbadessa G, Schwartz BE, Kazakin J, Droz Dit Busset M, Shaib WL. ARQ 087, an oral pan-fibroblast growth factor receptor (FGFR) inhibitor, in patients (pts) with advanced intrahepatic cholangiocarcinoma (iCCA) with FGFR2 genetic aberrations. J Clin Oncol. 2017;35(15_suppl):4017. doi: 10.1200/JCO.2017.35.15_suppl.4017. [DOI] [Google Scholar]
- 64.Javle M, Bekaii-Saab T, Jain A, Wang Y, Kelley RK, Wang K, Kang HC, Catenacci D, Ali S, Krishnan S, Ahn D, Bocobo AG, Zuo M, Kaseb A, Miller V, Stephens PJ, Meric-Bernstam f, Shroff R, Ross J. Biliary cancer: utility of next-generation sequencing for clinical management. Cancer. 2016;122(24):3838–3847. doi: 10.1002/cncr.30254. [DOI] [PubMed] [Google Scholar]
- 65.Jusakul A, Cutcutache I, Yong CH, Lim JQ, Huang MN, Padmanabhan N, Nellore V, Kongpetch S, Ng AWT, Ng LM, Choo SP, Myint SS, Thanan R, Nagarajan S, Lim WK, Ng CCY, Boot A, Liu M, Ong CK, Rajasegaran V, Lie S, Lim AST, Lim TH, Tan J, Loh JL, McPherson JR, Khuntikeo N, Bhudhisawasdi V, Yongvanit P, Wongkham S, Totoki Y, Nakamura H, Arai Y, Yamasaki S, Chow PK, Chung AYF, Ooi LLPJ, Lim KH, Dima S, Duda DG, Popescu I, Broet P, Hsieh SY, Yu MC, Scarpa A, Lai J, Luo DX, Carvalho AL, Vettore AL, Rhee H, Park YN, Alexandrov LB, Gordân R, Rozen SG, Shibata T, Pairojkul C, Teh BT, Tan P. Whole-genome and epigenomic landscapes of etiologically distinct subtypes of cholangiocarcinoma. Cancer Disc. 2017;7(10):1116–1135. doi: 10.1158/2159-8290.CD-17-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Verlingue L, Malka D, Allorant A, Massard C, Ferté C, Lacroix L, Rouleau E, Auger N, Ngo M, Nicotra C, De Baere T, Tselikas L, Ba B, Michiels S, Scoazec JY, Boige V, Ducreux M, Soria JC, Hollebecque A. Precision medicine for patients with advanced biliary tract cancers: an effective strategy within the prospective MOSCATO-01 trial. Eur J Cancer. 2017;87:122–130. doi: 10.1016/j.ejca.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 67.Valle JW, Furuse J, Jitlal M, Baere S, Mizuno N, Wasan H, Bridgewater J, Okusaka T. Cisplatin and gemcitabine for advanced biliary tract cancer: a meta-analysis of two randomised trials. Ann Oncol. 2014;25(2):391–398. doi: 10.1093/annonc/mdt540. [DOI] [PubMed] [Google Scholar]
- 68.Vogel A, Kasper S, Bitzer M, Block A, Sinn M, Schulze-Bergkamen H, Moehler M, Pfarr N, Endris V, Goeppert B, Merx K, Schnoy E, Siveke JT, Michl P, Waldschmidt D, Kuhlmann J, Geissler M, Kahl C, Evenkamp R, Schmidt T, Kuhlmann A, Weichert W, Kubicka S. PICCA study: panitumumab in combination with cisplatin/gemcitabine chemotherapy in KRAS wild-type patients with biliary cancer – a randomised biomarker- driven clinical phase II AIO study. Eur J Cancer. 2018;92:11–19. doi: 10.1016/j.ejca.2017.12.028. [DOI] [PubMed] [Google Scholar]
- 69.Lamarca A, Barriuso J, McNamara MG, Valle JW. Biliary tract Cancer: state of the art and potential role of DNA damage repair. Cancer Treat Rev. 2018;70:168–177. doi: 10.1016/j.ctrv.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 70.Bogenberger JM, DeLeon TT, Arora M, Ahn DH, Borad MJ. Emerging role of precision medicine in biliary tract cancers. NPJ Precis Oncol. 2018;2:21. doi: 10.1038/s41698-018-0064-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lowery MA, Ptashkin R, Jordan E, Berger MF, Zehir A, Capanu M, Kemeny NE, O’Reilly EM, El-Dika I, Jarnaging WR, Harding JJ, D’Angelica MI, Cercek A, Hechtman JF, Solit DB, Schultz N, Hyman DM, Klimstra DS, Saltz LB, Abou-Alfa GK. Comprehensive molecular profiling of intrahepatic and extrahepatic cholangiocarcinomas: potential targets for intervention. Clin Cancer Res. 2018;24(17):4154–4161. doi: 10.1158/1078-0432.CCR-18-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lowery MA, Abou-Alfa GK, Burris HA, Janku F, Shroff RT, Cleary JM, Azad NS, Goyal L, Maher EA, Gore L, Hollebecque A, Beeram M, Trent JC, Jiang L, Ishii Y, Auer J, Gliser C, Agresta SV, Pandya SS, Zhu AW. Phase I study of AG-120, an IDH1 mutant enzyme inhibitor: results from the cholangiocarcinoma dose escalation and expansion cohorts. J Clin Oncol. 2017;35(15_suppl):4015. doi: 10.1200/JCO.2017.35.15_suppl.4015. [DOI] [Google Scholar]
- 73.Abou-Alfa GK, Macarulla Mercade T, Javle M, Kelley RK, Lubner S, Adeva J, Cleary JM, Catenacci DV, Borad MJ, Bridgewater JA, Harris WP, Murphy AG, Oh D-Y, Whisenant J, Wu B, Jiang L, Gliser C, Pandya SS, Valle JW, Zhu AW. LBA10_PRClarIDHy: A global, phase III, randomized, doubleblind study of ivosidenib (IVO) vs placebo in patients with advanced cholangiocarcinoma (CC) with an isocitrate dehydrogenase 1 (IDH1) mutation. Ann Oncol. 2019;30(Suppl_5) doi: 10.1093/annonc/mdz394.027. [DOI] [Google Scholar]
- 74.Catarino R, Coelho A, Araújo A, Gomes M, Nogueira A, Lopes C, Medeiros R. Circulating DNA: diagnostic tool and predictive marker for overall survival of NSCLC patients. PLoS One. 2012;7:e38559. doi: 10.1371/journal.pone.0038559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pantel K, Alix-Panabières C. Real-time liquid biopsy in cancer patients: fact or fiction. Cancer Res. 2013;73(21):6384–6388. doi: 10.1158/0008-5472.CAN-13-2030. [DOI] [PubMed] [Google Scholar]
- 76.Alix-Panabieres C, Pantel K. Circulating tumor cells liquid biopsy of cancer. Clin Chem. 2013;59:110–118. doi: 10.1373/clinchem.2012.194258. [DOI] [PubMed] [Google Scholar]
- 77.Schwarzenbach H, Hoon DSB, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer. 2011;11:426–437. doi: 10.1038/nrc3066. [DOI] [PubMed] [Google Scholar]
- 78.Bedard PL, Hansen AR, Ratain MJ, Siu LL. Tumour heterogeneity in the clinic. Nature. 2013;501:355–364. doi: 10.1038/nature12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alix-Panabières C, Pantel K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov. 2016;6(05):479–491. doi: 10.1158/2159-8290.CD-15-1483. [DOI] [PubMed] [Google Scholar]
- 80.Haber DA, Velculescu VE. Blood-based analyses of cancer: circulating tumor cells and circulating tumor DNA. Cancer Discov. 2014;4(06):650–666. doi: 10.1158/2159.8290.CD-13-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ciccarese C, Santoni M, Brunelli M, Buti S, Modena A, Nabissi M, Artibani W, Martignoni G, Montironi R, Tortora G, Massari F. AR-V7 and prostate cancer: The watershed for treatment selection. Cancer Treat Rev. 2016;43:27–35. doi: 10.1016/j.ctrv.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 82.van de Stolpe A, Pantel K, Sleijfer S, Terstappen LW, den Toonder JM. Circulating tumor cell isolation and diagnostics: toward routine clinical use. Cancer Res. 2011;71:5955–5960. doi: 10.1158/0008-5472.CAN-11-1254. [DOI] [PubMed] [Google Scholar]
- 83.Crowley E, Di Nicolantonio F, Loupakis F, Bardelli A. Liquid biopsy: monitoring cancer-genetics in the blood. Nat Rev Clin Oncol. 2013;10:472–484. doi: 10.1038/nrclinonc.2013.110. [DOI] [PubMed] [Google Scholar]
- 84.Gold B, Cankovic M, Furtado LV, Meier F, Gocke CD. Do circulating tumor cells, exosomes, and circulating tumor nucleic acids have clinical utility? A report of the Association for Molecular Pathology. J Mol Diagn. 2015;17:209–224. doi: 10.1016/j.jmoldx.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nedaeinia R, Manian M, Jazayeri MH, Ranjbar M, Salehi R, Sharifi M, Mohaghegh F, Goli M, Jahednia SH, Avan A, Ghayour-Mobarhan M. Circulating exosomes and exosomal microRNAs as biomarkers in gastrointestinal cancer. Cancer Gene Ther. 2017;24:48–56. doi: 10.1038/cgt.2016.77. [DOI] [PubMed] [Google Scholar]
- 86.Mader S, Pantel K. Liquid biopsy: Current status and future perspectives. Oncol Res Treat. 2017;40(7-8):404–408. doi: 10.1159/000478018. [DOI] [PubMed] [Google Scholar]
- 87.Palmirotta R, Lovero D, Silvestris E, Felici C, Quaresmini D, Cafforio P, Silvestris F. Next-generation sequencing (NGS) analysis on single circulating tumor cells (CTCs) with no need of whole-genome amplification (WGA) Cancer Genomics Proteomics. 2017;14(3):173–179. doi: 10.21873/cgp.20029.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mandel P, Metais P. Les acides nucleiques du plasma sanguin chez l'homme. C R Seances Soc Biol Fil. 1948;142:241–243. [PubMed] [Google Scholar]
- 89.Leon SA, Shapiro B, Sklaroff DM, Yaros MJ. Free DNA in the serum of cancer patients and the effect of therapy. Cancer Res. 1977;37:646–650. [PubMed] [Google Scholar]
- 90.Normanno N, Cervantes A, Ciardiello F, De Luca A, Pinto C. The liquid biopsy in the management of colorectal cancer patients: Current applications and future scenarios. Cancer Treat Rev. 2018;70:1–8. doi: 10.1016/j.ctrv.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 91.Palmirotta R, Lovero D, Cafforio P, Felici C, Mannavola F, Pellè E, Quaresmini D, Tucci M, Silvestris F. Liquid biopsy of cancer: a multimodal diagnostic tool in clinical oncology. Ther Adv Med Oncol. 2018;10:1758835918794630. doi: 10.1177/1758835918794630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Marusyk A, Polyak K. Tumor heterogeneity: causes and consequences. Biochim Biophys Acta. 2010;1805:105–117. doi: 10.1016/j.bbcan.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chang JW, Shih CL, Wang CL, Luo JD, Wang CW, Hsieh JJ, Yu CJ, Chiou CC. Transcriptomic analysis in liquid biopsy identifies circulating PCTAIRE-1 mRNA as a biomarker in NSCLC. Cancer Genomics Proteomics. 2020;17(1):91–100. doi: 10.21873/cgp.20170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rossi G, Mu Z, Rademaker AW, Austin LK, Strickland KS, Costa RLB, Nagy RJ, Zagonel V, Taxter TJ, Behdad A, Wehbe FH, Platanias LC, Gradishar WJ, Cristofanilli M. Cell-free DNA and circulating tumor cells: Comprehensive liquid biopsy analysis in advanced breast cancer. Clin Cancer Res. 2018;24(3):560–568. doi: 10.1158/1078-0432.CCR-17-2092. [DOI] [PubMed] [Google Scholar]
- 95.Bergerot PG, Hahn AW, Bergerot CD, Jones J, Pal SK. The role of circulating tumor DNA in renal cell carcinoma. Curr Treat Opt Oncol. 2018;19:10. doi: 10.1007/s11864-018-0530-4. [DOI] [PubMed] [Google Scholar]
- 96.Siravegna G, Marsoni S, Siena S, Bardelli A. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 2017;14:531–548. doi: 10.1038/nrclinonc.2017.14. [DOI] [PubMed] [Google Scholar]
- 97.Cohen JD, Javed AA, Thoburn C, Wong F, Tie J, Gibbs P, Schmidt CM, Yip-Schneider MT, Allen PJ, Schattner M, Brand RE, Singhi AD, Petersen GM, Hong SM, Kim SC, Falconi M, Doglioni C, Weiss MJ, Ahuja N, He J, Makary MA, Maitra A, Hanash SM, Dal Molin M, Wang Y, Li L, Ptak J, Dobbyn L, Schaefer J, Silliman N, Popoli M, Goggins MG, Hruban RH, Wolfgang CL, Klein AP, Tomasetti C, Papadopoulos N, Kinzler KW, Vogelstein B, Lennon AM. Combined circulating tumor DNA and protein biomarker-based liquid biopsy for the earlier detection of pancreatic cancers. Proc Natl Acad Sci USA. 2017;114(38):10202–10207. doi: 10.1073/pnas.1704961114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen K, Zhang H, Zhang L, SQ J, Qi J, Huang DF, Li F, Wei Q, Zhang J. Value of circulating cell-free DNA in diagnosis of hepatocellular carcinoma. World J Gastroenterol. 2013;19(20):3143–3149. doi: 10.3892/mmr.2018.9336.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ellinger J, Wittkamp V, Albers P, Perabo FG, Mueller SC, Von Ruecker A, Bastian PJ. Cell-free circulating DNA: diagnostic value in patients with testicular germ cell cancer. J Urol. 2009;181:363–371. doi: 10.1016/j.juro.2009.02.106. [DOI] [PubMed] [Google Scholar]
- 100.Usiakova Z, Mikulova V, Pinterova D, Brychta M, Valchar J, Kubecova M, Tesarova P, Bobek V, Kolostova K. Circulating tumor cells in patients with breast cancer: Monitoring chemotherapy success. In Vivo. 2014;28(4):605–614. [PubMed] [Google Scholar]
- 101.Diehl F, Schmidt K, Choti MA, Romans K, Goodman S, Li M, Thornton K, Agrawal N, Sokoll L, Szabo SA, Kinzler KW, Vogelstein B, Diaz LA Jr. Circulating mutant DNA to assess tumor dynamics. Nat Med. 2008;14:985–990. doi: 10.1038/nm.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Murtaza M, Dawson SJ, Tsui DW, Gale D, Forshew T, Piskorz AM, Parkinson C, Chin SF, Kingsbury Z, Wong AS, Marass F, Humphray S, Hadfield J, Bentley D, Chin TM, Brenton JD, Caldas C, Rosenfeld N. Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature. 2013;497:108–112. doi: 10.1038/nature12065. [DOI] [PubMed] [Google Scholar]
- 103.Hao YX, Fu Q, Guo YY, Ye M, Zhao HX, Wang Q, Peng XM, Li QW, Wang RL, Xiao WH. Effectiveness of circulating tumor DNA for detection of KRAS gene mutations in colorectal cancer patients: a meta-analysis. Onco. Targets Ther. 2017;10:945–953. doi: 10.2147/OTT.S123954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Merker JD, Oxnard GR, Compton C, Diehn M, Hurley P, Lazar AJ, Lindeman N, Lockwood CM, Rai AJ, Schilsky RL, Tsimberidou AM, Vasalos P, Billman BL, Oliver TK, Bruinooge SS, Hayes DF, Turner NC. Circulating tumor DNA analysis in patients with cancer: American society of clinical oncology and college of American pathologists joint review. Arch Pathol Lab Med. 2018;142:1242–1253. doi: 10.5858/arpa.2018-0901-SA. [DOI] [PubMed] [Google Scholar]
- 105.Iijima Y, Hirotsu Y, Amemiya K, Ooka Y, Mochizuki H, Oyama T, Nakagomi T, Uchida Y, Kobayashi Y, Tsutsui T, Kakizaki Y, Goto T, Miyashita Y, Omata M. Very early response of circulating tumour–derived DNA in plasma predicts efficacy of nivolumab treatment in patients with non-small cell lung cancer. Eur J Cancer. 2017;86:349–357. doi: 10.1016/j.ejca.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 106.Ball MW, Gorin MA, Guner G, Pierorazio PM, Netto G, Paller CJ, Hammers HJ, Diaz LA, Allaf ME. Circulating tumor DNA as a marker of therapeutic response in patients with renal cell carcinoma: A pilot study. Clin Genitourin Cancer. 2016;14:e515–e520. doi: 10.1016/j.clgc.2016.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pal SK, Sonpavde G, Agarwal N, Vogelzang NJ, Srinivas S, Haas NB, Signoretti S, McGregor BA, Jones J, Lanman RB, Banks KC, Choueiri TK. Evolution of circulating tumor DNA profile from First-line to subsequent therapy in metastatic renal cell carcinoma. Eur Urol. 2017;72:557–564. doi: 10.1016/j.eurouro.2017.03.046. [DOI] [PubMed] [Google Scholar]
- 108.Li X, Ren R, Ren S, Chen X, Cai W, Zhou F, Zhang Y, Su C, Zhao C, Li J, Cheng N, Zhao M, Zhou C. Peripheral blood for epidermal growth factor receptor mutation detection in non-small cell lung cancer patients. Transl Oncol. 2014;7:341–348. doi: 10.1016/j.tranon.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mok T, Wu YL, Lee JS, Yu CJ, Sriuranpong V, Sandoval-Tan J, Ladrera G, Thongprasert S, Srimuninnimit V, Liao M, Zhu Y, Zhou C, Fuerte F, Margono B, Wen W, Tsai J, Truman M, Klughammer B, Shames DS, Wu L. Detection and dynamic changes of EGFR mutations from circulating tumor DNA as a predictor of survival outcomes in NSCLC Patients treated with first-line intercalated erlotinib and chemotherapy. Clin Cancer Res. 2015;21:3196–3203. doi: 10.1158/1078-0432.CCR-14-2594. [DOI] [PubMed] [Google Scholar]
- 110.Oxnard GR, Paweletz CP, Kuang Y, Mach SL, O'Connell A, Messineo MM, Luke JJ, Butaney M, Kirschmeier P, Jackman DM, Jänne PA. Noninvasive detection of response and resistance in EGFR-mutant lung cancer using quantitative next-generation genotyping of cell-free plasma DNA. Clin Cancer Res. 2014;20:1698–1705. doi: 10.1158/1078-0432.CCR-13-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cimadamore A, Gasparrini S, Santoni M, Cheng L, Lopez-Beltran A, Battelli N, Massari F, Giunchi F, Fiorentino M, Scarpelli M, Montironi R. Biomarkers of aggressiveness in genitourinary tumors with emphasis on kidney, bladder, and prostate cancer. Expert Rev Mol Diagn. 2018;18:645–655. doi: 10.1080/14737159.2018.1490179. [DOI] [PubMed] [Google Scholar]
- 112.Russo M, Siravegna G, Blaszkowsky LS, Corti G, Crisafulli G, Ahronian LG, Mussolin B, Kwak EL, Buscarino M, Lazzari L, Valtorta E, Truini M, Jessop NA, Robinson HE, Hong TS, Mino-Kenudson M, Di Nicolantonio F, Thabet A, Sartore-Bianchi A, Siena S, Iafrate AJ, Bardelli A, Corcoran RB. Tumor heterogeneity and lesion-specific response to targeted therapy in colorectal cancer. Cancer Discov. 2016;6:147–153. doi: 10.1158/2159-8290.CD-15-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ghidini M, Pizzo C, Botticelli A, Hahne JC, Passalacqua R, Tomasello G, Petrelli F. Biliary tract cancer: current challenges and future prospects. Cancer Manag Res. 2019;11:379–388. doi: 10.2147/CMAR.S157156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tamada K, Ushio J, Sugano K. Endoscopic diagnosis of extrahepatic bile duct carcinoma: Advances and current limitations. World J Clin Oncol. 2011;2:203–216. doi: 10.5306/wjco.v2.i5.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zou S, Li J, Zhou H, Frech C, Jiang X, Chu JS, Zhao X, Li Y, Li Q, Wang H, Hu J, Kong G, Wu M, Ding C, Chen N, Hu H. Mutational landscape of intrahepatic cholangiocarcinoma. Nat Commun. 2014;5:5696. doi: 10.1038/ncomms6696. [DOI] [PubMed] [Google Scholar]
- 116.Zill OA, Greene C, Sebisanovic D, Siew LM, Leng J, Vu M, Hendifar AE, Wang Z, Atreya CE, Kelley RK, Van Loon K, Ko AH, Tempero MA, Bivona TG, Munster PN, Talasaz A, Collisson EA. Cell-free DNA next-generation sequencing in pancreatobiliary carcinomas. Cancer Discov. 2015;5:1040–1048. doi: 10.1158/2159-8290.CD-15-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kumari S, Tewari S, Husain N, Agarwal A, Pandey A, Singhal A, Lohani M. Quantification of circulating free DNA as a diagnostic marker in gall bladder cancer. Pathol Oncol Res. 2017;23:91–97. doi: 10.1007/s12253-016-0087-0. [DOI] [PubMed] [Google Scholar]
- 118.Andresen K, Boberg KM, Vedeld HM, Honne H, Jebsen P, Hektoen M, Wadsworth CA, Clausen OP, Lundin KE, Paulsen V, Foss A, Mathisen Ø, Aabakken L, Schrumpf E, Lothe RA, Lind GE. Four DNA methylation biomarkers in biliary brush samples accurately identify the presence of cholangiocarcinoma. Hepatology. 2015;61(05):1651–1659. doi: 10.1002/hep.27707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Eaton JE, Gossard AA, Talwalkar JA. Recall processes for biliary cytology in primary sclerosing cholangitis. Curr Opin Gastroenterol. 2014;30(03):287–294. doi: 10.1097/MOG.0000000000000055. [DOI] [PubMed] [Google Scholar]
- 120.Andersen RF, Jakobsen A. Screening for circulating RAS/RAF mutations by multiplex digital PCR. Clin Chim Acta. 2016;458:138–143. doi: 10.1016/j.cca.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 121.Mody K, Kasi PM, Yang Y, Surapaneni PK, Bekaii-Saab T, Ahn DH, Mahipal A, Sonbol MB, Starr JS, Roberts A, Nagy R, Lanman R, Borad MJ. Circulating tumor DNA profiling of advanced biliary tract cancers. JCO Precis Oncol. 2019;3(3):1–9. doi: 10.1200/PO.18.00324. [DOI] [PubMed] [Google Scholar]
- 122.Lucci A, Hall CS, Lodhi AK, Bhattacharyya A, Anderson AE, Xiao L, Bedrosian I, Kuerer HM, Krishnamurthy S. Circulating tumour cells in non-metastatic breast cancer: a prospective study. Lancet Oncol. 2012;13:688–695. doi: 10.1016/S1470-2045(12)70209-7. [DOI] [PubMed] [Google Scholar]
- 123.Ansari J, Yun JW, Kompelli AR, Moufarrej YE, Alexander JS, Herrera GA, Shackelford RE. The liquid biopsy in lung cancer. Genes Cancer. 2016;7(11-12):355–367. doi: 10.18632/genesandcancer.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ettrich TJ, Schwerdel D, Dolnik A, Beuter F, Blätte TJ, Schmidt SA, Stanescu-Siegmund N, Steinacker J, Marienfeld R, Kleger A, Bullinger L, Seufferlein T, Berger AW. Genotyping of circulating tumor DNA in cholangiocarcinoma reveals diagnostic and prognostic information. Sci Rep. 2019;9(1):13261. doi: 10.1038/s41598-019-49860-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Churi CR, Shroff R, Wang Y, Rashid A, Kang HC, Weatherly J, Zuo M, Zinner R, Hong D, Meric-Bernstam F, Janku F, Crane CH, Mishra L, Vauthey JN, Wolff RA, Mills G, Javle M. Mutation profiling in cholangiocarcinoma: prognostic and therapeutic implications. PLoS One. 2014;9(12):e115383. doi: 10.1371/journal.pone.0115383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lamarca A, Barriuso J, McNamara MG, Valle JW. Molecular targeted therapies: ready for "prime time" in biliary tract cancer. J Hepatol. 2020;pii:S0168–8278(20)30165-3. doi: 10.1016/j.jhep.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 127.Filippi R, Lombardi P, Quarà V, Fenocchio E, Aimar G, Milanesio M, Leone F, Aglietta M. Pharmacotherapeutic options for biliary tract cancer: current standard of care and new perspectives. Expert Opin Pharmacother. 2019;20(17):2121–2137. doi: 10.1080/14656566.2019.1667335. [DOI] [PubMed] [Google Scholar]
- 128.Goyal L, Saha SK, Liu LY, Siravegna G, Leshchiner I, Ahronian LG, Lennerz JK, Vu P, Deshpande V, Kambadakone A, Mussolin B, Reyes S, Henderson L, Sun JE, Van Seventer EE, Gurski JM Jr, Baltschukat S, Schacher-Engstler B, Barys L, Stamm C, Furet P, Ryan DP, Stone JR, Iafrate AJ, Getz G, Porta DG, Tiedt R, Bardelli A, Juric D, Corcoran RB, Bardeesy N, Zhu AX. Polyclonal secondary FGFR2 mutations drive acquired resistance to FGFR inhibition in patients with FGFR2 fusion-positive cholangiocarcinoma. Cancer Discov. 2017;7(3):252–263. doi: 10.1158/2159-8290.CD-16-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shen N, Zhang D, Yin L, Qiu Y, Liu J, Yu W, Fu X, Zhu B, Xu X, Duan A, Chen Z, Wang X, Cao X, Zhao T, Zhou Z, Yu L, Qin H, Fang Z, Li JY, Liu Y, Xiong L, Yuan B, Li F, Zhang Y. Bile cell free DNA as a novel and powerful liquid biopsy for detecting somatic variants in biliary tract cancer. Oncol Rep. 2019;42(2):549–560. doi: 10.3892/or.2019.7177. [DOI] [PMC free article] [PubMed] [Google Scholar]