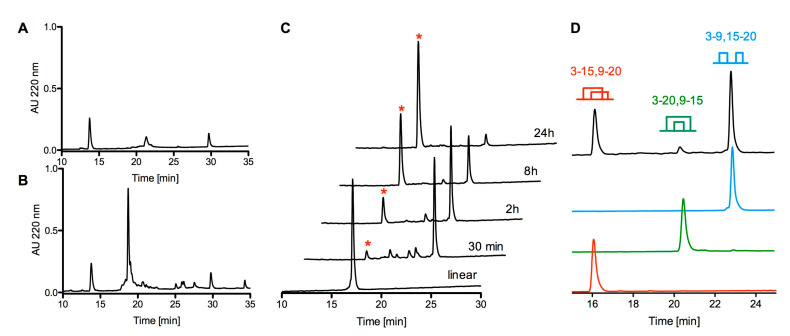
Figure 5.
Chemical synthesis and folding of cdg14a. (A) HPLC chromatogram of a crude linear cdg14a eluted on an analytical C18 column using a gradient ranging from 5% to 65% solvent B in 30 min with 1 mL/min flow rate. (B) HPLC chromatogram of a crude linear cdg14a modified with 2-aminoethyl methanethiosulfonate hydrobromide (MTSEA) (HPLC conditions are the same as in panel A). (C) oxidative folding of cdg14a monitored by HPLC, using a C18 column and a gradient ranging from 15% to 45% solvent B, with a flow rate 1 mL/min. Single asterisk denotes the native-like fold of cdg14a. (D) zoom-in on 4 h oxidative folding time point (black HPLC trace) with all possible folding isomers identified at 220 nm. The red trace represents the native folding isomer with the globular-like connectivity Cys3-Cys15, Cys9-Cys20; green trace represents ribbon-like folding isomer with the Cys3-Cys20, Cys9-Cys15 connectivity; blue trace represents the fast folding product with the bead-like connectivity Cys3-Cys9, Cys15-Cys20. The traces were collected using the same gradient as described for panel (C).