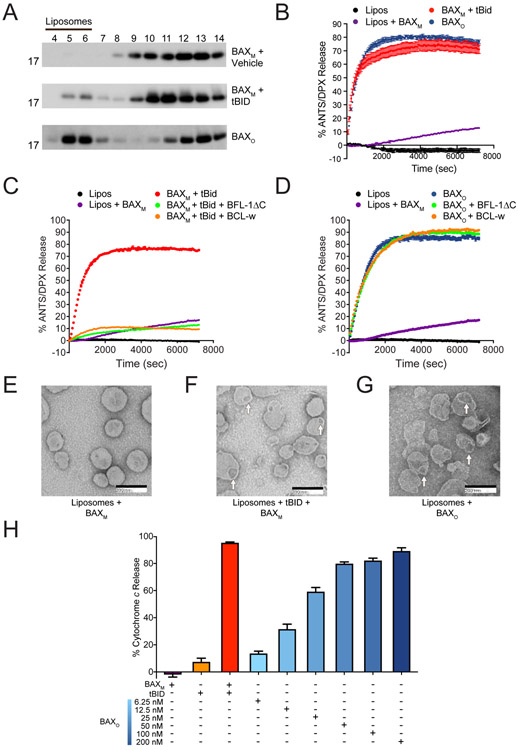
Figure 2. The biochemical activity of BAXO emulates BH3-triggered BAX.
(A) BAXO and tBID-triggered BAXM translocate to liposomes, whereas vehicle-treated BAXM remains in the soluble fraction, as assessed at 1 h by liposomal translocation assay and BAX western analysis. BAXO and BAXM, 500 nM; tBid, 20 nM. Data shown are representative of two independent biological replicates. Fractions: liposomal, 4-6; supernatant, 7-14.
(B) BAXO and tBID-triggered BAXM induce liposomal poration with similar kinetics and potency, as assessed by liposomal release assay. Error bars are mean ± s.e.m. for experiments performed in technical triplicate, with data representative of two independent experiments. BAXM, 500 nM; BAXO, 500 nM (equivalent total protein amounts); tBID, 20 nM.
(C-D) Anti-apoptotic proteins effectively blocked liposomal poration induced by BAXM upon tBID triggering (C), but had no inhibitory effect on BAXO (D). Error bars are mean ± s.e.m. for experiments performed in technical triplicate, with data representative of two independent experiments. BAXM and BAXO 500 nM; tBID, 20 nM; anti-apoptotic proteins, 10 μM.
(E-G) Negative stain EM of liposomes incubated with BAXM (E), tBID-triggered BAXM (F), or BAXO (G), showing similar morphology of membrane disruption and pore size induced by BAXO and BH3-triggered BAXM after 15 min. Mean pore diameter (nm) ± s.e.m.: tBID-triggered BAX, 48.9 ± 2.3; BAXo, 56.2 ± 2.6; p value = 0.195.
(H) BAXO induces dose-responsive cytochrome c release from BAX/BAK-deficient mouse liver mitochondria (MLM). tBID-triggered BAX release is shown as a positive control. Error bars are mean ± s.e.m. for experiments performed in technical triplicate, with data representative of two independent experiments. BAXM, 200 nM; BAXO, 6.25-200 nM; tBID, 40 nM.
See also Figures S3 and S4.