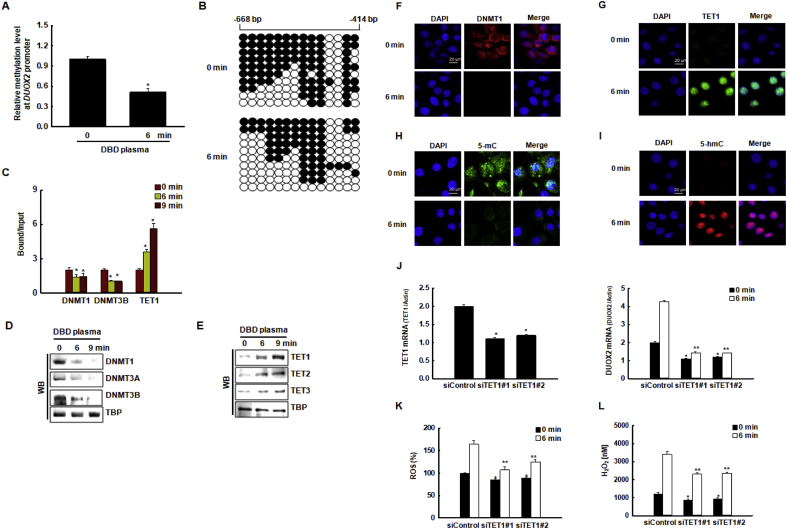
Fig. 5.
DBD plasma-induced transcriptional upregulation of DUOX2 correlates with DNA demethylation of the DUOX2 promoter region. (A) Relative methylation levels of the DUOX2 promoter region were normalized to the Alu element in cells exposed to DBD plasma for 0 min or 6 min *p < 0.05 indicates a statistically significant difference between DUOX2 promoter methylation in cells exposed to DBD plasma for 6 min (“6” min) and non-exposed cells (“0” min). (B) Bisulfite sequencing analysis of the DUOX2 promoter region in cells exposed to DBD plasma for 0 min or 6 min was assessed. Black circles represent methylated cytosine resides; white circles represent unmethylated cytosine residues. (C) Cells exposed to DBD plasma for 0, 6, or 9 min. The binding of DNMT1, DNMT3B, and TET1 to the DUOX2 promoter was assessed by chromatin immunoprecipitation coupled with quantitative PCR. *p < 0.05 indicates a statistically significant difference between the binding of DNMT1, DNMT3B, and TET1 to the DUOX2 promoter in DBD plasma-exposed cells (“6” and “9” min) and the non-exposed cells (“0” min), respectively. The nuclear fractions of the cells were electrophoresed, and (D) the expression of DNMT1, DNMT3A, and DNMT3B, and (E) the expression of TET1, TET2, and TET3 were detected by western blotting. TBP was used as the loading control. The nuclear localization of (F) DNMT1 and (G) TET1 was confirmed by confocal microscopy. Scale bars = 20 μm. The effect of DBD plasma on TET1 activity, i.e., the conversion of (H) 5-mC to (I) 5-hmC, was assessed by confocal microscopy. Scale bars = 20 μm. (J, left graph) The relative expression of TET1 mRNA and (J, right graph) DUOX2 mRNA in cells transfected with TET1-specific siRNAs or with control siRNAs were assessed by qRT-PCR. *p < 0.05 indicates a statistically significant difference between the expression of TET1 mRNA in cells transfected with siTET1 and cells transfected with siControl. *, **p < 0.05 indicates a statistically significant difference between the expression of DUOX2 mRNA in cells transfected with siTET1 and the cells transfected with siControl at 0 min and 6 min, respectively. (K) ROS levels were detected by DCF-DA staining and flow cytometry. *, **p < 0.05 indicates a statistically significant difference between the expression of ROS levels in cells transfected with siTET1 and the cells transfected with siControl at 0 min and 6 min, respectively. (L) H2O2 concentrations were assessed using the Amplex® red reagent. *, **p < 0.05 indicates a statistically significant difference between the expression of H2O2 concentrations in cells transfected with siTET1 and the cells transfected with siControl at 0 min and 6 min, respectively. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)