Abstract
While COVID-19 pandemic continues to affect our country and most countries in the world, we have to make some changes both in our social life and our approach to healthcare. We have to struggle with the pandemic on one hand and also try to follow up and treat our patients with chronic diseases in the most appropriate way. In this period, one of our group of patients who are challenging us for follow-up and treatment are those who should start or continue to use immunosuppressive therapy. In order to contribute to the accumulation of knowledge in this area, we wanted to report a patient who was followed up with the diagnosis of COVID-19 and had been administered rituximab very recently due to a nephrotic syndrome caused by membranous nephropathy.
Keywords: Coronavirus, COVID-19, Immunosuppression, Membranous nephropathy, Rituximab
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-related coronavirus disease-19 (COVID-19) cases, which emerged in Wuhan [1], China in December 2019 and soon became a pandemic [2], started to appear in our country in March 2020. Membranous nephropathy (MN) is one of the most important causes of nephrotic syndrome (NS) in adults [3]. For treatment of MN, either cyclophosphamide or cyclosporin-A has been used in combination with glucocorticoids for many years. However, recently, rituximab has started to come to the fore [4]. There is insufficient data about COVID-19’s course in immunocompromised patients, especially in NS patients using immunosuppressive agents. As far as we know, COVID-19 has not been reported in glomerulonephritis patients who use immunosuppressives. Herein, a case of COVID-19 who was recently treated with rituximab for MN is presented.
Case presentation
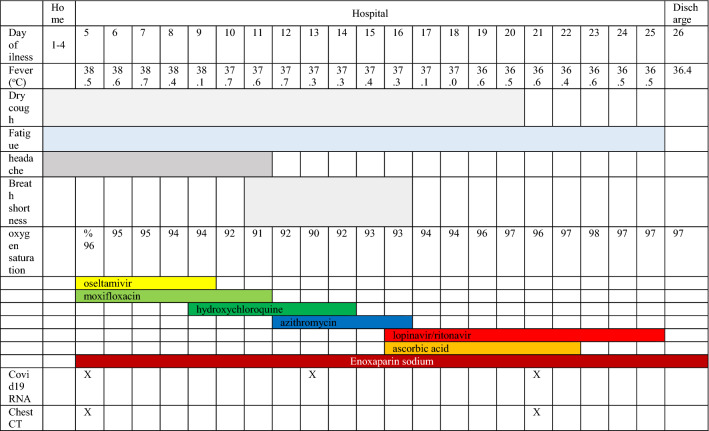
A 48-year old male who was admitted to the emergency clinic with cough, high fever and headache for 4 days was hospitalized on March 15, 2020 with a pre-diagnosis of COVID 19 pneumonia. He is non smoker and not use alcohol, and working as a parking guard. At the latest outpatient visit on March 04, there were no signs or symptoms of infection. His past medical history included type 2 diabetes since 2013. Hemoglobin A1C of 5.8% on March 04, 2020. There was no evidence of retinopathy in the ocular examination and no neuropathy. A renal biopsy performed due to nephrotic syndrome (proteinuria: 8330 mg/day) in May 2019 revelaed MN. At the time of diagnosis, serum albumin and creatinine was 2.4 g/dL and 0.7 mg/dL, respectively. Secondary MN causes were eliminated and primary MN was diagnosed after M-type anti-phospholipase A2 receptor antibodies were positive (715 RU/mL). The patient was started on valsartan and furosemide for proteinuria and edema, and continued to use gliclazide and metformin. There was no significant decrease in proteinuria, 1 g rituximab was administered for 2 times (day 0 and 15) in January and February 2020. On the last follow-up, the patient was not in any kind of remission. Physical examination at presentation to the emergency room revealed that he was febrile (38.5 °C) and normotensive (110/70 mmHg), his pulse was 96 min and rhythmic, and oxygen saturation was 96% while breathing ambient air. On auscultation breath sounds were rough. No crepitations or rhonci were detected. He was empirically administered oseltamivir 75 mg BID and moxifloxacin 400 mg QD on March 15. Microbiological assessment was negative for viral, bacterial or fungal infections, including aspergillosis, pneumocystis, and mycobacteria. Laboratory parameters throughout the follow-up are shown in Table 1, and pneumonic infiltration on computed tomography (CT) is shown in Fig. 1a–d. Nasopharyngeal swab sample was negative for influenza. However, swabs for SARS-CoV 2 by real-time reverse-transcriptase–polymerase-chain-reaction (rRT-PCR) assay were found positive. Due to persistent high fevers, hydroxychloroquine (400 mg BID for 24 h, afterwards 200 mg BID; oral) was added to his treatment on March 19, and on March 21, moxifloxacin was discontinued and azithromycin was given for 5 days. There was no QT prolongation in the control electrocardiogram. C-reactive protein significantly increased to 116 mg/L (23 March). On March 26, lopinavir/ritonavir 200/50 mg 2*2 for 10 days and ascorbic acid 25,000 mg/50 mL QD for 7 days were added to the treatment regimen. As of March 29, his fever returned to normal and never increased again. Control swabs for SARS CoV-2 by rRT-PCR assay were reported as negative twice. During the follow-up, he just needed nasal oxygen and did not need intensive care. Serum creatinine decreased to 0.7 mg/dL from 1.6 mg/dL with proper hydration. During his follow-up, his blood sugar remained stable. No significant increase was observed. Enoxaparin sodium was given as an anticoagulant during hospital stay. Although his second chest CT (Fig. 1e–h) showed more severe pneumonia, he was decided to be discharged because of clinical and laboratory improvement on April 05. The clinical course of the patient is shown in Table 2.
Table 1.
Laboratory parameters throughout the follow-up
| Date | 15/03/20 | 18/03/20 | 21/03/20 | 23/03/20 | 26/03/20 | 29/03/20 | 01/04/20 | 03/04/20 | 13/05/20 |
|---|---|---|---|---|---|---|---|---|---|
| Test | |||||||||
| WBC (4500–11,000/μL) | 5610 | 2960 | 5160 | 6490 | 5510 | 4950 | 7210 | 7810 | 6890 |
| Neu (1500–7500/μL) | 2930 | 1690 | 4060 | 5340 | 4880 | 3720 | 5700 | 6200 | 4070 |
| Lym (600–3400 μ/L) | 2180 | 890 | 780 | 990 | 490 | 930 | 940 | 1060 | 1950 |
| Hb (13–17 g/dL) | 10.9 | 10.3 | 10.2 | 9.6 | 8.8 | 9.5 | 7.9 | 9.1 | 11.7 |
| PLT (142–424,000 μ/L) | 222,000 | 173,000 | 273,000 | 287,000 | 334,000 | 299,000 | 396,000 | 442,000 | 278,000 |
| CRP (0–5 mg/L) | 31.7 | 12.8 | 116.4 | 85.7 | 42.3 | 19 | 0.99 | ||
| Procalcitonin (0–0.5 ng/mL) | 0.85 | 0.89 | 0.5 | 0.25 | |||||
| ESR (0–20 mm/h) | 70 | 106 | 124 | 140 | 140 | 48 | |||
| Ferritin (22–274 ng/mL) | 586.9 | 1692 | 727 | 143 | |||||
| Glukoz (70–100 mg/dL) | 159 | 165 | 130 | 152 | 168 | 153 | |||
| Urea (19–44 mg/dL) | 57 | 37 | 43 | 35 | 32 | 27 | 23 | 39 | |
| Cr (0.7–1.25 mg/dL) | 1.6 | 1.2 | 1.26 | 1.24 | 1.03 | 0.87 | 0.76 | 1.27 | |
| eGFR (> 90 mL/dk/1.73 m2) | 49 | 70 | 67 | 68 | 85 | 101 | 107 | 66 | |
| AST (5–34 U/L) | 55 | 32 | 31 | 27 | 39 | 16 | 16 | 15 | |
| ALT (0–55 U/L) | 26 | 20 | 19 | 19 | 24 | 17 | 14 | 10 | |
| ALP (40/150 U/L) | 46 | 40 | 46 | ||||||
| GGT (12–64 U/L) | 22 | 18 | 16 | ||||||
| LDH (125–220 U/L) | 513 | 468 | 460 | 476 | 483 | 347 | 313 | ||
| CK (30–200 U/L) | 516 | 253 | 256 | 233 | 135 | ||||
| Ca (8.5–10.5 mg/dL) | 7.3 | 8.4 | 8.3 | 8.5 | 8.7 | ||||
| Mg (1.6–2.6 mg/dL) | 1.8 | ||||||||
| Na (135–145 mmol/L) | 139 | 139 | 135 | 137 | 138 | 137 | 142 | 138 | |
| K (3.5–5.1 mmol/L) | 2.66 | 3.3 | 3.1 | 2.9 | 3.2 | 3.45 | 4.2 | 4.2 | |
| Total protein (6.2–8 g/dL) | 4.6 | 4.7 | |||||||
| Albumin (3.5–5 g/dL) | 1.9 | 2.1 | |||||||
| PT (12–16.8 s) | 16.8 | 16.2 | |||||||
| aPTT (24–42 s) | 37.3 | 35 | |||||||
| Serum IgG (540–1822 mg/dL) | 545 | 638 | |||||||
| Serum IgA (63–484 mg/dL) | 240 | 251 | |||||||
| Serum IgM (22–240 mg/dL) | 37 | 41 | |||||||
ALP alkaline phosphatase, ALT alanine aminotransferase, AST aspartate aminotransferase, Ca calcium, CK creatine kinase, Cr creatinine, CRP c-reactive protein, eGFR estimated glomerular filtration rate, ESR erytrocyte sedimantation rate, GGT gamma glutamyl transferase, Hb hemoglobin, K potassium, LDH lactate dehydrogenase, Lym lymphocyte, Mg magnesium, Na sodium, Neu neutrophil, PLT platelet, PT protrombin time, WBC white blood cell
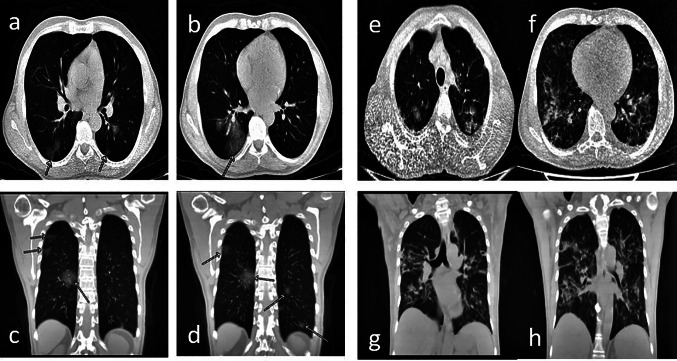
Fig. 1.
a–d Chest CT shows diffuse areas of diffuse ground-glass infiltrates in both lungs, scattered in a patchy style on March 15, 2020, after symptom onset. e–h Chest CT on March 31, 2020; In a comparative evaluation with CT of 15.03.2020, there is progression in pneumonic consolidations in both lungs. Interlobuler septal thickening in both lungs and pleural effusion increases reaching 10 mm on the left and 3 mm on the right in both hemithorax were observed as new findings
Table 2.
The clinical course of the patient
Discussion
According to World Health Organization data on June 18, 2020, there have been 8,242,999 cases of COVID-19 and 445,535 death in the whole world and 182,727 cases with 4861 deaths in Turkey [5]. According to these data, the average mortality is 5.4% in all over the World and 2.6% in Turkey. The most frequent symptoms are high fever, cough, myalgia and fatigue. More rarely, sputum, headache and diarrhea can be seen. Average incubation period is 5.2 days [6]. According to a meta-analysis, approximately 30% of patients require intensive care caused by acute respiratory distress syndrome, and case fatality rate is 6.8% [7]. Patients who need intensive care and result with mortality are mostly over 65 years of age or have additional comorbidities such as hypertension, chronic obstructive pulmonary disease and diabetes. Some patients have leukocytosis but more of them have leukopenia and lymphopenia, high CRP, LDH and erythrocyte sedimentation rate. CT generally shows bilateral pneumonic infiltration. The most common pattern is ground glass appearance [7]. Opportunistic infections are common in immunocompromised patients [8] and symptoms often become blurred even in typical infectious diseases in these patients. Also, the disease course tends to be heavier in these patients compared to healthy population [9]. We see this situation most frequently in patients with kidney transplants and glomerulonephritis, to whom we administer immunosuppressive therapy. Chronic kidney disease (CKD) patients, especially in end stage, are also considered to be severely immunocompromised [10]. COVID-19 is also expected to be severe in our patients on immunosuppressive therapy. However, our data on this issue is limited. Alberici et al. reported that 5 of 20 patients with kidney transplants diagnosed with COVID-19 died, and four required intensive care. Five of 21 hemodialysis patients died. Mean duration of hospital stay in discharged patients was 12–13 days. Two of the five patients with CKD died [11]. Also, several recovered COVID-19 patients with kidney transplants and a patient with liver transplant have also been reported [9, 12, 13]. In these cases, mycophenolate mofetil, tacrolimus and everolimus were temporarily discontinued in patients with kidney transplantation. In one case, tacrolimus was maintained with a low trough level aiming at 2–4.5 ng/mL [12]. While hydroxychloroquine, oseltamivir, azithromycin or moxifloxacin are generally used in treatment, corticosteroids, tocilizumab, intravenous immunoglobulin, remdecivir and favipravir are used for severe disease [14]. Different treatment protocols are applied in different countries. Although there have not been previously reported glomerulonephritis with COVID-19, patients who use immunosuppressive therapy due to rheumatological diseases may show similarity to our patients with NS. Monti et al. reported eight COVID-19 patients in a series of 320 cases diagnosed with rheumatoid arthritis and spondyloarthropathy. These patients had taken immunosuppressive drugs such as etanercept, abatacept, tofacitinib, baricitinib, methotrexate and leflunomide. Four of them had taken low-dose glucocorticoids and three of them hydroxychloroquine. Only a 65-year old patient needed oxygen and was hospitalized. No one died. Immunosuppressive treatments of all patients were discontinued from the onset of symptoms [15]. Again, it is recommended not to stop the immunosuppressive drugs used for rheumatological diseases during the COVID-19 pandemic by rheumatology specialists from Italy [16]. If the high mortality in kidney transplant and chronic dialysis patients is remembered, it can be understand how difficult this decision is about our patients. Coronavirus uses ACE2, an angiotensin I-converting enzyme (ACE) homologue, which is a cell surface receptor, to enter the cells. While this receptor is slightly expressed in endothelium and kidney, is particularly expressed in airways and heart [17]. Therefore, use of ACE inhibitors (ACEi) and angiotensin receptor blockers (ARBs) has been widely discussed since the beginning of the pandemic. However, clinical studies have shown that ACEi or ARBs do not increase morbidity or mortality in COVID-19 patients [18–20]. Our patient continued to use valsartan. Our patient with bilateral COVID-19 pneumonia who had severe NS due to MN and had received rituximab 1 month ago did not develop severe hypoxemia and did not need intensive care. However, high fever remained for a long time. Hospital stay was well above average (21 days). Combination of hydroxychloroquine, moxifloxacin and azithromycin, oseltamivir, and lopinavir/ritonavir were used at different times for treatment. Our experience in the monitoring and treatment of COVID-19 is increasing day by day. Recently, we have started to use hydroxychloroquine and azithromycin or moxifloxacin in the early onset of pneumonia. When hypoxemia starts to develop, we choose to start patients on favipiravir in order to avoid the need for intensive care. We give prophylactic anticoagulants to all inpatients. We believe that this treatment approach is associated with low mortality in our country [14]. In nephrology practice, there are situations where we cannot stop immunosuppressive treatment such as kidney transplantation and vasculitis leading to rapidly progressive glomerulonephritis. On the other hand, we have patients with NS, in whom immunosuppressive therapy can be delayed for a while. Some of them were started on immunosuppressive agents before the pandemic. Some of our patients which have NS also have an indication for immunosuppressive therapy during the pandemic. We are undecided about temporarily discontinuing the treatment of our patients currently on immunosuppressives agents or postponing the treatment of our patients in need for treatment. According to our observations, we concluded that the use of rituximab should be preferred in patients with absolute indication. Because, we did not observe any significant side effects or increase in mortality due to rituximab. As a result, we make individual decisions on a case-by-case basis. In order to make more accurate decisions, more data on this issue should be collected. Therefore, we thought that it was appropriate to report this case to contribute to this accumulating data.
Compliance with ethical standards
Conflict of interest
The authors have declared that no conflict of interest exists.
Informed consent
Informed consent was obtained from the patient included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lu H, Stratton CW, Tang Y-W. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol. 2020;92:401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicola M, O'Neill N, Sohrabi C, Khan M, Agha M, Agha R. Evidence based management guideline for the COVID-19 pandemic- review article. Int J Surg. 2020 doi: 10.1016/j.ijsu.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Couser WG. Primary membranous nephropathy. Clin J Am Soc Nephrol. 2017;12(6):983–997. doi: 10.2215/CJN.11761116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rojas-Rivera JE, Carriazo S, Ortiz A. Treatment of idiopathic membranous nephropathy in adults: KDIGO 2012, cyclophosphamide and cyclosporine A are out, rituximab is the new normal. Clin Kidney J. 2019;12(5):629–638. doi: 10.1093/ckj/sfz127.PMID:31583088;PMCID:PMC6768298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Coronavirus disease 2019 (COVID-19) situation report–150 https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200618covid-19-sitrep-150.pdf?sfvrsn=aa9fe9cf_2. Accessed 18 June 2020.
- 6.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao Y, Liu X, Xiong L, Cai KI. Imaging and clinical features of patients with 2019 novel coronavirus SARS- CoV- 2: a systematic review and meta- analysis. J Med Virol. 2020 doi: 10.1002/jmv.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bayraktar A, Catma Y, Akyildiz A, Demir E, Bakkaloglu H, Ucar AR, et al. Infectious complications of induction therapies in kidney transplantation. Ann Transplant. 2019;12(24):412–417. doi: 10.12659/AOT.915885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guillen E, Pineiro GJ, Revuelta I, Rodriguez D, Bodro M, Moreno A, et al. Case report of COVID-19 in a kidney transplant recipient: does immunosuppression alter the clinical presentation? Am J Transplant. 2020 doi: 10.1111/ajt.15874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin J, Tang W, Liu W, Yu F, Wu Y, Fang X, et al. Decreased B1 and B2 lymphocytes are associated with mortality in elderly patients with chronic kidney diseases. Front Med. 2020;20(7):75. doi: 10.3389/fmed.2020.00075.PMID:32266271;PMCID:PMC7098909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alberici F, Delbarba E, Manenti C, Econimo L, Valerio F, Alessandra P, et al. Management of patients on dialysis and with kidney transplant during SARS COV-2 (COVID-19) pandemic in Brescia, Italy. Kidney Int Rep. 2020 doi: 10.1016/j.ekir.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhong Z, Zhang Q, Xia H, Wang A, Liang W, Zhou W, et al. Clinical characteristics and immunosuppressants management of coronavirus disease 2019 in solid organ transplant recipients. Am J Transplant. 2020;20(7):1916–1921. doi: 10.1111/ajt.15928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu L, Xu X, Ma K, Yang J, Guan H, Chen S, et al. Successful recovery of COVID-19 pneumonia in a renal transplant recipient with long-term immunosuppression. Am J Transplant. 2020;20(7):1859–1863. doi: 10.1111/ajt.15869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Rapublic of Turkey Ministry of Health. Guidence to COVID-19 (SARS-CoV2 infection) (scientific board study) https://hsgm.saglik.gov.tr/depo/birimler/goc_sagligi/covid19/rehber/COVID-19_Rehberi20200414_eng_v4_002_14.05.2020.pdf. Accessed 18 Aug 2020.
- 15.Monti S, Balduzzi S, Delvino P, Bellis E, Quadrelli VS, Montecucco C. Clinical course of COVID-19 in a series of patients with chronic arthritis treated with immunosuppressive targeted therapies. Ann Rheum Dis. 2020;79(667):668. doi: 10.1136/annrheumdis-2020-217424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ceribelli A, Motta F, De Santis M, Ansari AA, Ridgway WM, Gershwin ME, Selmi C. Recommendations for coronavirus infection in rheumatic diseases treated with biologic therapy. J Autoimmun. 2020;109:102442. doi: 10.1016/j.jaut.2020.102442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rico-Mesa JS, White A, Anderson AS. Outcomes in patients with COVID-19 infection taking ACEI/ARB. Curr Cardiol Rep. 2020;22(5):31. doi: 10.1007/s11886-020-01291-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng YD, Meng K, Guan HQ, Leng L, Zhu RR, Wang BY, et al. Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019- nCoV. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:E004. doi: 10.3760/cma.j.cn112148-20200220-00105. [DOI] [PubMed] [Google Scholar]
- 19.Zhang P, Zhu L, Cai J, Lei F, Qin JJ, Xie J, et al. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020;126(12):1671–1681. doi: 10.1161/CIRCRESAHA.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh AK, Gupta R, Misra A. Comorbidities in COVID-19: outcomes in hypertensive cohort and controversies with renin angiotensin system blockers. Diabetes Metab Syndr. 2020;14(4):283–287. doi: 10.1016/j.dsx.2020.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]