Table 1. Optimization of the carboamination conditions a .
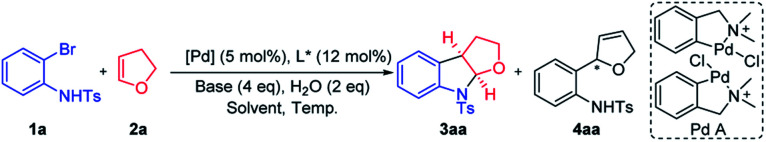
| |||||
| Entry | [Pd]/L | Base | Solvent/T (°C) | 3/4 | Yield b , c (ee) [%] |
| 1 | Pd2(dba)3/L3 | NaOtBu | DCM/100 | 5 : 1 | 73(47) |
| 2 | Pd2(dba)3/L3 | LiOtBu | DCM/100 | — | Trace |
| 3 | Pd2(dba)3/L3 | KOtBu | DCM/100 | — | Mix |
| 4 | Pd2(dba)3/L3 | NaOEt | DCM/100 | 2 : 1 | 52(40) |
| 5 | Pd2(dba)3/L3 | NaOPh | DCM/100 | 2 : 1 | 63(78) |
| 6 | Pd2(dba)3/L3 | NaOPh | MTBE/100 | 1 : 1 | 44(60) |
| 7 | Pd2(dba)3/L3 | NaOPh | 1,2-DCE/100 | 9 : 1 | 81(76) |
| 8 | Pd2(dba)3/L3 | NaOPh | Toluene/100 | 1 : 1 | 42(53) |
| 9 | Pd2(dba)3/L3 | NaOPh | MeOH/100 | 1 : 1 | 39(59) |
| 10 | Pd2(dba)3/L4 | NaOPh | 1,2-DCE/100 | 9 : 1 | 78(87) |
| 11 | Pd2(dba)3/L5 | NaOPh | 1,2-DCE/100 | >30 : 1 | 81(93) |
| 12 | Pd2(dba)3/L6 | NaOPh | 1,2-DCE/100 | — | Trace |
| 13 | Pd2(dba)3/L7 | NaOPh | 1,2-DCE/100 | 15 : 1 | 77(77) |
| 14 | Pd2(dba)3/L8 | NaOPh | 1,2-DCE/100 | >30 : 1 | 83(93) |
| 15 | Pd(dba)2/L8 | NaOPh | 1,2-DCE/100 | >30 : 1 | 79(94) |
| 16 | Pd2(dba)3·CHCl3/L8 | NaOPh | 1,2-DCE/100 | >30 : 1 | 81(94) |
| 17 | Pd(OAc)2/L8 | NaOPh | 1,2-DCE/100 | >30 : 1 | 74(94) |
| 18 | (η3-C3H5)2Pd2Cl2/L8 | NaOPh | 1,2-DCE/100 | >30 : 1 | 69(94) |
| 19 | Pd A/L8 | NaOPh | 1,2-DCE/100 | >30 : 1 | 82(94) |
| 20 | Pd A/L8 | NaOPh | 1,2-DCE/80 | >30 : 1 | 81(93) |
| 21 | Pd A/L8 | NaOPh | 1,2-DCE/50 | >30 : 1 | 81(95) |
| 22 | Pd A/L8 | NaOPh | 1,2-DCE/20 | >30 : 1 | 84(96) |
| 23 d | Pd A/L8 | NaOPh | 1,2-DCE/20 | >30 : 1 | 79(96) |
aUnless otherwise specified, all reactions were carried out with 1a (0.2 mmol), 2a (0.8 mmol, 4 eq.), a [Pd] source (0.01 mmol, 5 mol%), N-Me-Xiang-phos (0.024 mmol, 12 mol%), base (0.8 mmol, 4 eq.), and H2O (7.2 μL, 2 eq.) in a solvent (1 mL, 0.2 M).
bYield of isolated product 3aa.
cDetermined by chiral HPLC.
d2 eq. H2O were removed.
