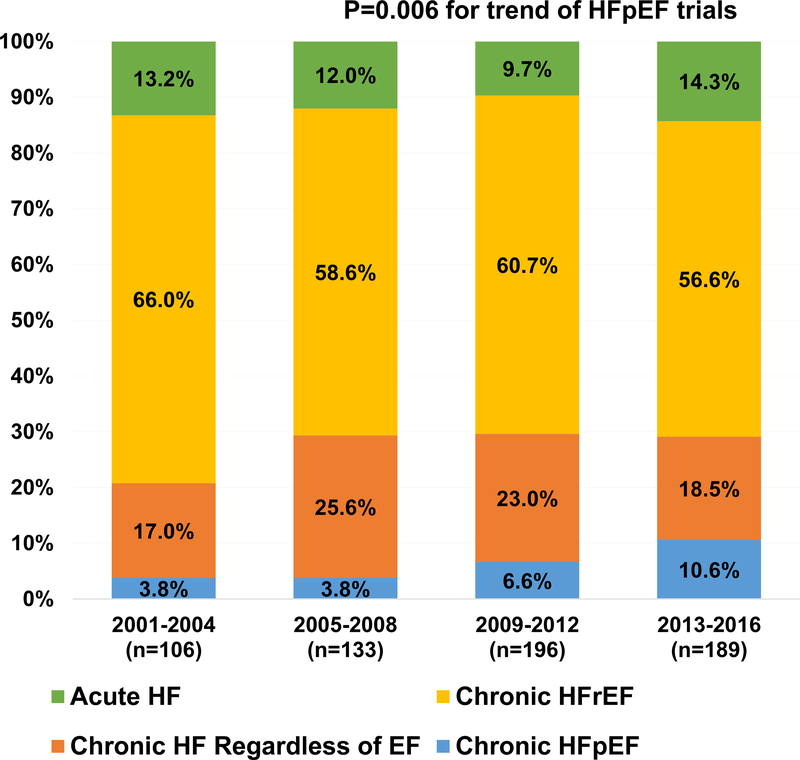
Despite numerous clinical trials, there remain no definitively proven therapies for patients with heart failure with preserved ejection fraction (HFpEF). While this experience may be in part related to the intervention tested itself, certain other factors related to the design of the clinical trial itself, including size, conduct, enrollment, endpoint selection, and funding source may impact the results.(1) Furthermore, the representation and the temporal trends of participation by the elderly, women, and racial/ethnic minorities in HFpEF clinical trials has not been well characterized. Systematic evaluation of these trial-level factors may inform future design of efficient and effective drug and device development programs in HFpEF.
Accordingly, we conducted a comprehensive systematic review of operational and patient-level characteristics of contemporary HFpEF trials published over the last 16 years (January 2001 and December 2016) using 2 strategies; a) PubMed/MEDLINE query with the following limits: publication year, “heart failure”, “trial*”, and “randomized” and b) ClinicalTrials.gov query limited to adult, interventional, phase II-IV, heart failure trials. We excluded phase I, pilot trials, secondary, interim, or post hoc analyses. The following data were abstracted: (1) year of publication; (2) intervention; (3) enrollment duration; (4) subjects enrolled; (5) number of participating centers and countries; (6) ejection fraction (EF) cut-offs; (7) mean age of the cohort (8) proportion of women enrolled (9) race and ethnicity when reported (10) primary endpoints; and (11) fundingsources. Based on ClinicalTrials.gov designations, funding source was classified as (1) industry; (2) government; (3) university or other non-profit or non-federal organizations. Primary endpoints were classified as mortality, intermediate (e.g., quality of life, dyspnea relief, hospitalization, length of stay), or surrogate (e.g., pulmonary capillary wedge pressure, natriuretic peptides, etc.). Regions of enrollment were divided into: (1) exclusively North America (NA); (2) exclusively Western Europe (WE); (3) exclusively outside of NA and WE - rest of the world (RoW); and (4) mixed/multi-regional. We compared these clinical trial data with epidemiologic data in the US (weighted for sample size) collected from large registries or observational studies of patients with HFpEF (2–5).
We screened 5,488 studies and identified 624 HF clinical trials of which 374 (60%) studied HF with reduced EF (HFrEF), 42 (7%) focused on HFpEF, 132 (21%) trials enrolled HF participants regardless of their EF, and 76 (12%) trials were conducted in the acute HF setting. Overall, these trials collectively enrolled 12,185 patients from a total of 946 sites; 16 trials enrolled more than 100 subjects. The number of HFpEF trials increased from 4 in 2001–2004 to 21 in 2013–2016 (P=0.006 for trend, Figure 1). Key characteristics of HFpEF trials are provided in Table 1. The left ventricular EF cut-off to define HFpEF increased from a median 45% in trials conducted in 2001–2008 to 50% in 2009–2016. Median numbers of participants and enrolling sites per HFpEF trial were significantly lower than for HFrEF and acute HF trials, and remained stable over time (P>0.05 for both trends). No differences were observed in regional distribution of HFpEF trials over time (P=0.30).
Figure 1.
Trends in HF by EF and hospitalization status over 16-year study period.
Table 1.
Trial-Level Characteristics of HF Trials Published from 2001 to 2016
| Trial characteristics | Chronic HFpEF trials with >100 patients (n=16) | All Chronic HFpEF trials (n=42) | Chronic HFrEF trials (n=374) | Chronic HF regardless of EF (n=132) | Acute HF trials (n=76) | P-Value* |
|---|---|---|---|---|---|---|
| Year published | ||||||
| 2001–2008 | 5 (31) | 8 (19) | 148 (40) | 52 (40) | 30 (39) | |
| 2009–2016 | 11 (69) | 34 (81) | 226 (60) | 80 (61) | 46 (61) | 0.14 |
| Trial size, median (interquartile range) | ||||||
| Patients | 220 (147–365) | 62 (30–162) | 97.5 (41–335) | 141.5 (65–277) | 106.5 (55.5–326.5) | 0.03 |
| Sites | 39 (12–65) | 1 (1–12) | 4 (1–38) | 2 (1–7) | 8 (1–31) | 0.002 |
| Countries | 2 (1–8) | 1 (1–2) | 1 (1–2) | 1 (1–1) | 1 (1–2) | <0.001 |
| Duration (years) | 3 (2.2–4.8) | 2.3 (1.3–4.2) | 2.1 (1.42–3.33) | 1.92 (1.17–3.08) | 2 (1.17–2.74) | 0.23 |
| Enrollment rate (patient/site/month) | 0.29 (0.18–0.57) | 0.88 (0.39–2.23) | 0.61 (0.26–2.0) | 2.25 (1.13–4.23) | 0.68 (0.42–2.02) | <0.001 |
| Ejection fraction cutoff (%) | 45 (45–50) | 50 (45–50) | 40 (35–40) | - | - | <0.001 |
| Intervention | ||||||
| Device | 0 (0) | 0 (0) | 64 (17) | 3 (2) | 5 (7) | |
| Medication | 15 (94) | 34 (81) | 180 (48) | 43 (33) | 57 (75) | |
| Others | 1 (6) | 8 (19) | 93 (25) | 80 (61) | 5 (7) | <0.001 |
| Procedure | 0 (0) | 0 (0) | 23 (6) | 3 (2) | 7 (9) | |
| Surgery | 0 (0) | 0 (0) | 7 (2) | 1 (1) | 1 (1) | |
| Testing/Imaging | 0 (0) | 0 (0) | 7 (2) | 2 (2) | 1 (1) | |
| Sponsor | ||||||
| Government | 6 (38) | 17 (40) | 75 (20) | 32 (24) | 9 (12) | |
| University/Organization | 1 (6) | 10 (24) | 92 (25) | 52 (39) | 25 (33) | <0.001 |
| Industry | 9 (56) | 14 (33) | 153 (41) | 28 (21) | 32 (42) | |
| Region | ||||||
| Rest of the world | 3 (23) | 7 (18) | 60 (17) | 34 (26) | 15 (20) | 0.003 |
| Multiregional | 3 (23) | 4 (10) | 69 (19) | 5 (4) | 16 (22) | |
| North America | 3 (23) | 15 (39) | 103 (29) | 46 (35) | 23 (31) | |
| Western Europe | 4 (31) | 13 (33) | 126 (35) | 46 (35) | 20 (27) | |
| Multicenter (>3 sites) | 13 (93) | 21 (50) | 178 (50) | 47 (37) | 41 (54) | |
| Multinational | 10 (67) | 13 (32) | 112 (30) | 9 (7) | 22 (29) | <0.001 |
| Primary Outcome | ||||||
| Mortality | 4 (25) | 4 (10) | 102 (27) | 38 (29) | 12 (16) | |
| Non-mortality intermediate outcomes | 8 (50) | 21 (50) | 141 (38) | 63 (48) | 25 (33) | 0.001 |
| Surrogate outcomes | 4 (25) | 17 (40) | 130 (35) | 31 (23) | 39 (51) |
Comparison across trials of chronic HFpEF, chronic HFrEF, chronic HF regardless of EF, and acute HF.
Data reported as n (%) or median (interquartile range).
Abbreviations: EF = ejection fraction; HF = heart failure; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction.
The weighted mean age of participants in HFpEF trials was 70±9 years compared with the corresponding average age of 73 years of patients with HFpEF in US epidemiologic studies. There was a trend towards enrolling younger participants in HFpEF trials over time (72±7 years in 2001–2008 vs. 68±9 years in 2009–2016). Overall, women constituted 55% of participants; this proportion trended down from 59% in 2001–2008 to 53% in 2009–2016. Trials conducted exclusively in North America enrolled a higher proportion of women (63%), which is consistent with the estimated proportion of 62% in US epidemiologic studies, compared with trials conducted exclusively in Western Europe (51%).(2–5)
Overall, 10% of participants in studies reporting race/ethnicity distribution data (n=37) were non-White, which increased from 7% in 2001–2008 to 13% in 2009–2016. North American trials included 21% participants of non-White race, which is lower than the proportion reported in US epidemiologic studies of 26–34%. Trials with sample sizes over 100 were less likely to enroll non-White patients (10%) compared with smaller trials (19%). Only 7 studies (17%) reported Black race which constituted 6% of patients enrolled in these studies (increased from 2% in 2001–2008 to 10% in 2009–2016) (Supplemental Table 1, Supplemental Figure 1).
Overall, mean body mass index in HFpEF trials was 30.3±2.3 which increased from 27.5±1.0 in trials conducted in 2001–2008 to 30.8±2.1 in 2009–2016. Highest body mass index was observed in trials conducted in North America with mean of 33.2±2.9 compared with 31.0±0.4 in multiregional trials, 29.0±1.4 in Western Europe, and 27.9±3.2 in trials conducted in RoW (P=0.002).
Data to calculate enrollment rate were available in 34 trials (81%). Median enrollment rate among HFpEF trials was 0.88 (inter-quartile range 0.39–2.23) patients/site/month, similar to that observed in HFrEF and acute HF trials, but lower than those in HF trials without EF-specific cut-offs (P<0.001) (Table 1). Enrollment rate in HFpEF trials enrolling more than 100 participants was significantly lower (median 0.29, inter-quartile range 0.18–0.57 patient/site/month) compared with smaller HFpEF trials (median 1.47, inter-quartile range 0.85–2.59; P<0.001). Enrollment rates in HFpEF trials stratified by key trial-level characteristics are demonstrated in Supplemental Table 2.
This comprehensive systematic review highlights important aspects of HFpEF trial design and research methodology over a 16-year period. Although the proportion of HFpEF trials conducted over time has increased gradually and these programs represent only ~10% of the contemporary HF trial enterprise. Most HFpEF trials are small, single-center experiences with non-mortality outcomes, and almost exclusively test pharmacotherapies. Elderly and female patients are historically underrepresented in cardiovascular clinical trials however, these data suggest that the prevalence of these subsets in contemporary HFpEF trials did not markedly differ from those seen in US epidemiologic studies. Although the proportion of non-White participants among clinical trials for HFpEF appears to be increasing over time, not all trials provided a detailed breakdown of race/ethnicity data, and therefore, we may have overestimated the proportion of non-White participants. Adequate enrollment of these minority populations is essential to ensure the generalizability of study findings to all patients with HFpEF.
In the present study, the median enrollment rate in larger (N>100) HFpEF trials was less than half that in chronic HFrEF trials. Among all HFpEF trials, risk factors for slow enrollment correlated with the scale and complexity of the trial program, with multicenter, multinational, and trial size >100 patients being associated with particularly slow enrollment. Despite large trials likely devoting more trial resources and having greater potential to influence clinical practice, our findings suggest these larger programs are in more need of targeted initiatives to improve enrollment rates. Methods to better identify high-quality sites and improve enrollment incentives are needed to augment efficient patient recruitment, which may potentially shorten trial duration and improve the generalizability of study results. Specific strategies may include use of a pre-trial registry, registry-based trials, application of pragmatic trial designs with less restrictive selection criteria, provision of incentives for recruitment, and improvement in patient and provider awareness of clinical research.
We observed significant heterogeneity in terms of EF thresholds adopted in HFpEF trials which increased from a median of 45% to 50% over the study period, which is in keeping with contemporary guidelines in defining HFpEF. Standardization of the HFpEF definition used for research purposes is essential to reduce heterogeneity in this patient population.
There are few large ongoing or planned advanced-phase trials in HFpEF: PARAGON-HF (NCT01920711); EMPEROR-Preserved (NCT03057951); and SPIRRIT (NCT02901184). These trials are anticipated to enroll over 3,000 patients each and will expand our current understanding of this syndrome. There has been little progress in device or procedure innovation for HFpEF or in evaluating interventions targeting patients hospitalized for HFpEF.
This trial-level systematic analysis represents a comprehensive characterization of the HFpEF clinical trial enterprise over the last 16 years and highlights an important mismatch between a limited research pipeline and the large burden of disease. Although the number of trials is slowly increasing over time, the majority of completed HFpEF trials were limited in size and scope and were not equipped to expand the therapeutic armamentarium for a significant proportion of this population. Enrollment rates across HFpEF programs remains suboptimal, lower than other HF trials, and particularly low among large, multicenter, and multinational trials. There is an unmet need to improve the future execution of HFpEF trial programs to ensure such therapies are well tested and that the evidence generated is generalizable.
Supplementary Material
Acknowledgments
Disclosures: Dr. Samman Tahhan is supported by the Abraham J. & Phyllis Katz Foundation (Atlanta, GA) and NIH/NIA grant AG051633. Dr. Muthiah Vaduganathan is supported by the NHLBI T32 postdoctoral training grant (T32HL007604). Dr. Stephen J. Greene is supported by the NHLBI T32 postdoctoral training grant (T32HL069749–14) and a Heart Failure Society of America/Emergency Medicine Foundation Acute Heart Failure Young Investigator Award funded by Novartis. Dr. Javed Butler is a principal investigator of the EMPEROR program (Boehringer Ingelheim); has received research support from the National Institutes of Health and European Union; and has been a consultant for Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, CVRx, Janssen, Luitpold, Medtronic, Merck, Novartis, Relypsa, StealthPeptide, Vifor, and ZS Pharma. All other authors have no conflicts to declare.
REFERENCES
- 1.Samman Tahhan A, Vaduganathan M, Kelkar A et al. Trendsin Heart Failure Clinical Trials From 2001–2012. J Card Fail 2016;22:171–9. [DOI] [PubMed] [Google Scholar]
- 2.Gerber Y, Weston SA, Redfield MM et al. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med 2015;175:996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gottdiener JS, McClelland RL, Marshall R et al. Out come of congestive heart failure in elderly persons: influence of left ventricular systolic function. The Cardiovascular Health Study. Ann Intern Med 2002;137:631–9. [DOI] [PubMed] [Google Scholar]
- 4.Lee DS, Gona P, Vasan RS et al. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the framingham heart study of the national heart, lung, and blood institute. Circulation 2009;119:3070–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silverman MG, Patel B, Blankstein R et al. Impact of Race, Ethnicity, and Multimodality Biomarkers on the Incidence of New-Onset Heart Failure With Preserved Ejection Fraction (from the Multi-Ethnic Study of Atherosclerosis). Am J Cardiol 2016;117:1474–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.