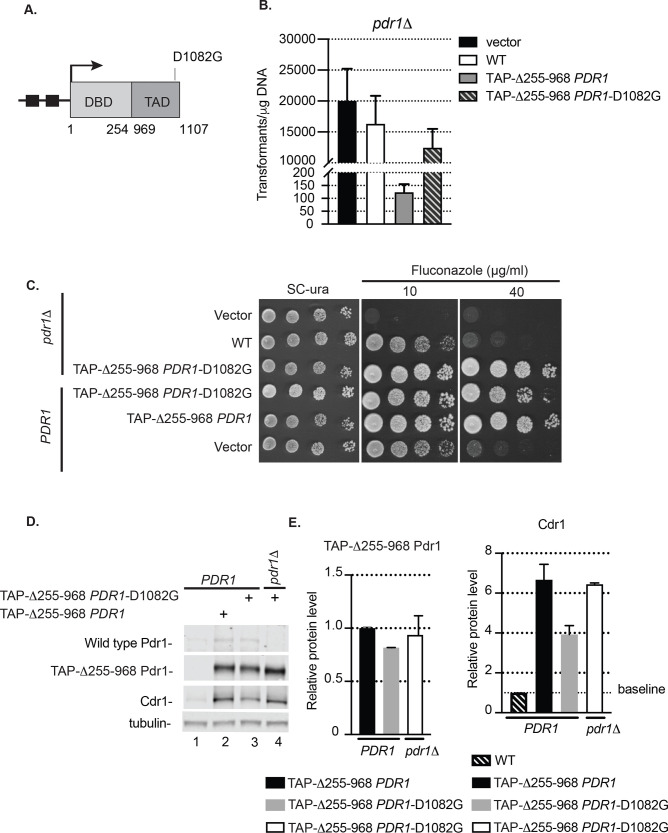
Fig 4. Insertion of D1082G mutation abolishes the toxicity of PDR1 lacking the central regulatory domain in pdr1Δ cells.
A. Diagram of PDR1 gene encoding the internal deletion Δ255–968 Pdr1 derivative with D1082G mutation. Functional domains (DBD, DNA binding domain and TAD, transactivation domain) of the protein and relative location of gain-of-function mutation D1082G is indicated. See legend to Fig 1 for further details. B. Transformation efficiency of vector alone or carrying wild type PDR1 or internal deletion PDR1 forms in pdr1Δ cells. Note discontinuous ordinate. C. Wild type (PDR1) and pdr1Δ strain transformed with vector (v) alone or carrying various Pdr1 forms were grown to mid-log phase and tested for fluconazole susceptibility in YPD media containing 10 or 40 μg/ml of drug. D. Mid-log cells expressing internal deletion Pdr1 forms Δ255–968 PDR1 or Δ255–968 PDR1-D1082G in wild type (PDR1) or pdr1Δ background were grown in minimal selective media, subjected to protein extraction and analyzed for Pdr1, TAP-Δ255–968 Pdr1 and Cdr1 levels by western blotting using anti-Pdr1 or anti-Cdr1 antibodies. Tubulin was used as loading control. A representative western blot is shown. E. Relative TAP-Δ255–968 Pdr1 and Cdr1 protein level in wild type (PDR1) or pdr1Δ strain expressing various Pdr1 forms. Levels of TAP-Δ255–968 Pdr1 in strains were normalized to wild type strain expressing also TAP-Δ255–968 Pdr1. Levels of Cdr1 were normalized to Cdr1 driven from wild type strain. Error bars represent standard error of the mean.