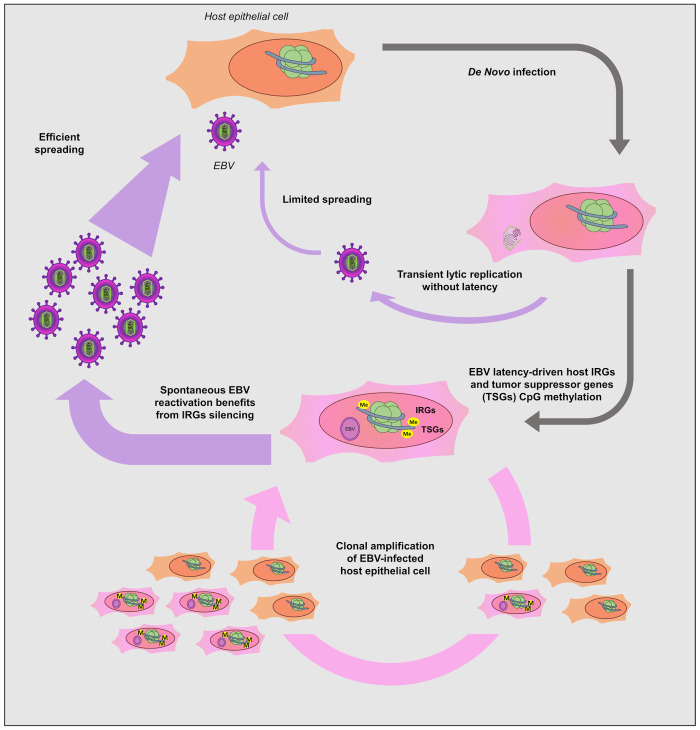
Fig 8. A working model describing the epigenetic silencing of antiviral IRGs and tumor suppressor genes by EBV through DNA hypermethylation.
Once EBV infects the targeted host cells, i.e. gastric epithelium, host DNA methylation machineries are activated, leading to DNA hypermethylation across host genome. Certain gene groups are preferentially subjected to such EBV-mediated epigenetic dysregulation, including tumor suppressors and our newly characterized IRGs. Silencing of tumor suppressor genes (TSGs) would of course promote tumor development, while silencing of IRGs, particularly those possessing antiviral activities, demonstrated by our studies, would benefit EBV viral propagation and spreading. Besides, silencing of IRGs may also benefit EBV oncogenesis, since some IRGs are reported to exert antitumor potential using the same immune signaling as its antiviral function. Therefore, massive DNA methylation of IRG loci would bring dual benefits to EBV, an oncovirus, not only favoring EBV lytic replication but also promoting malignant transformation of infected cells. The antiviral activities of some IRGs may not just limit to EBV, but extend to KSHV, another gamma-herpesvirus.