Table 2. Selection of diamidine analogues with aromatic linkers.
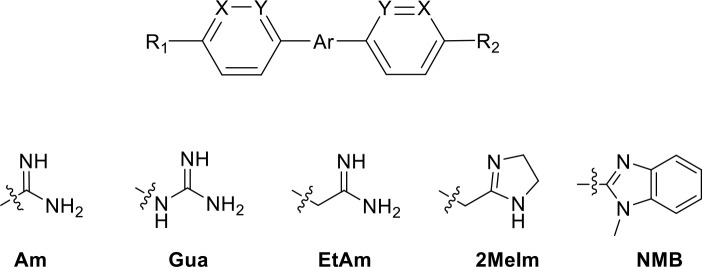 | |||||||
|---|---|---|---|---|---|---|---|
| Compound | R1 | R2 | Ar | X | Y | Ki
(µM) |
δ(ΔG0) PMD (kJ/mol) |
| DB75 | Am | Am | 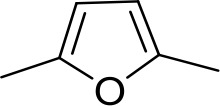 |
CH | CH | 38.2 ± 10.2 | 17.3 |
| DB607 | Am | OCH3 | 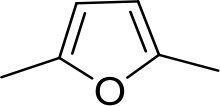 |
CH | CH | 18.1 ± 1.9 | 15.5 |
| DB960 | Am | NMBa | 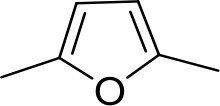 |
CH | CH | 16.6 ± 3.5 | 15.3 |
| DB994 | Am | Am |  |
N | CH | 167 ± 20 | 21.0 |
| DB829 | Am | Am |  |
CH | N | 39.9 ± 8.0 | 17.4 |
| DB1061 | EtAm | EtAm |  |
CH | CH | 32.3 ± 6.0 | 16.9 |
| DB1062 | 2MeIm | 2MeIm |  |
CH | CH | 59.6 ± 11.2 | 18.4 |
| ER1004 | Am | Am | 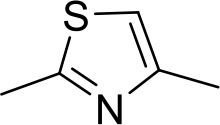 |
CH | CH | 68.7 ± 16.0 | 18.8 |
| DB320 | Am | Am | 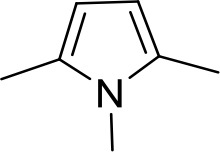 |
CH | CH | 71.3 ± 12.1 | 18.9 |
| DB686 | Gua | Gua | 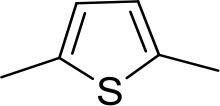 |
CH | CH | 0.29 ± 0.11 | 5.2 |
| DB1063 | EtAm | EtAm | 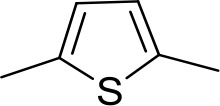 |
CH | CH | 0.40 ± 0.10 | 6.0 |
| DB1064 | 2MeIm | 2MeIm | 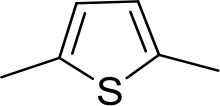 |
CH | CH | 3.0 ± 0.82 | 11.0 |
| DB1213 | Am | Am | 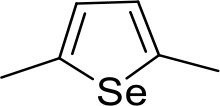 |
CH | CH | 0.72 ± 0.17 | 7.5 |
| DB1077 | Am | Am | 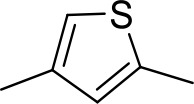 |
CH | CH | 13.8 ± 3.1 | 14.8 |
| DB914 | Am | Am | 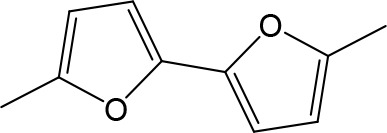 |
CH | CH | 0.073 ± 0.013 | 1.8 |
Am, amidine; Im, imidazole; EtAm, ethylamidine; 2MeIm, 2-methylimidazoline; NMB, N-methyl benzimidazole. aThis compound lacks the second benzene ring and features the terminal NMB moiety instead. Ki is the inhibition constant for [3H]-pentamidine transport by TbAQP2/HAPT1. δ(ΔG0) PMD is the difference in Gibbs Free Energy of interaction of the substrate with TbAQP2 with the same value for pentamidine (PMD). All Ki values are the average and SEM of at least 3–4 independent experiments.
