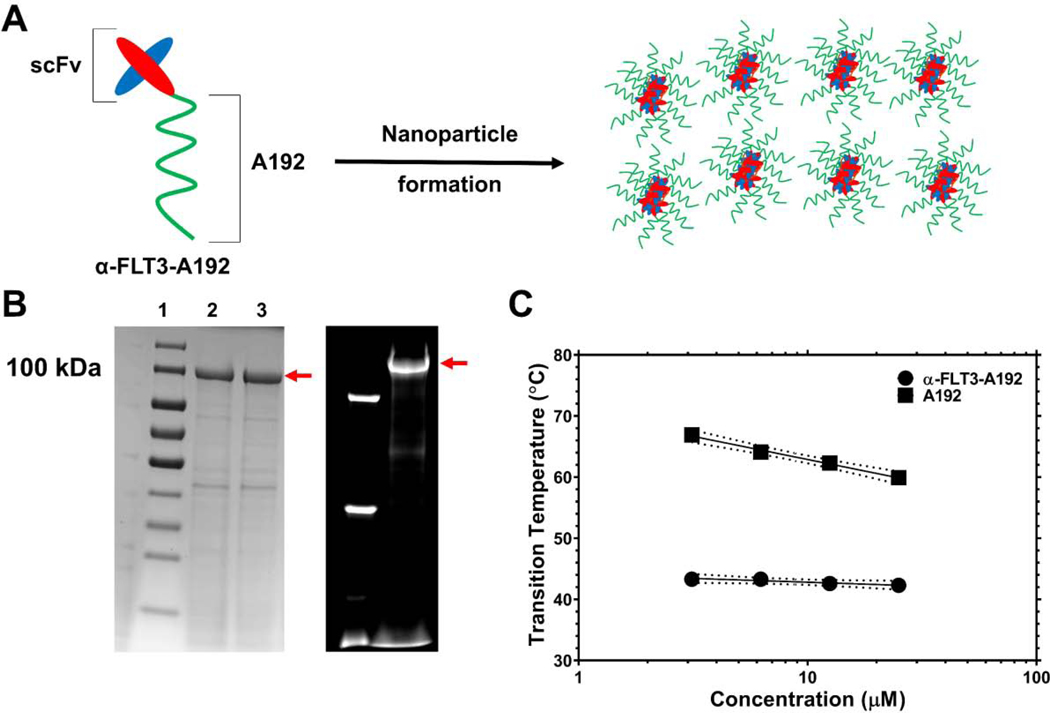
Figure 1. Characterization of an elastin-like polypeptide (ELP) fusion protein targeting the FLT3 receptor tyrosine kinase.
A) A single-chain variable fragment (scFv) targeting the FLT3 receptor tyrosine kinase was genetically fused to the amino-terminus of a high molecular weight ELP, A192 and expressed in E. coli. Dynamic light scattering (DLS) and size exclusion chromatography with multi-angle light scattering (SEC-MALS) suggest that α-FLT3-A192 forms nanoparticles. B) The recombinant fusion protein was purified using ELP-mediated phase separation, which was induced by increasing temperature and sodium chloride concentration. Using cycles of ‘cold’ and ‘hot’ centrifugation, recombinant fusion protein was obtained at high purity as demonstrated by SDS-PAGE stained by Coomassie. The red arrow indicates the major band for α-FLT3-A192. Lanes 2, 3 show the sample after 2, 3 purification cycles respectively. The purity of the major band in Lane 3 was estimated at 81.8%. On the right side, the fluorescent imaging of an SDS-PAGE gel shows the results of rhodamine labeling of α-FLT3-A192 used for PK study. The purity of the major band observed in the fluorescent imaging was estimated at 92.3 %. C) Optical density (350 nm) was used to evaluate ELP phase behavior over a range of temperatures and concentrations. While attachment of the scFv reduces the phase diagram curve with respect to free A192, at physiological salt concentrations (PBS) these ELP nanoparticles are expected to remain soluble during circulation at 37 °C.