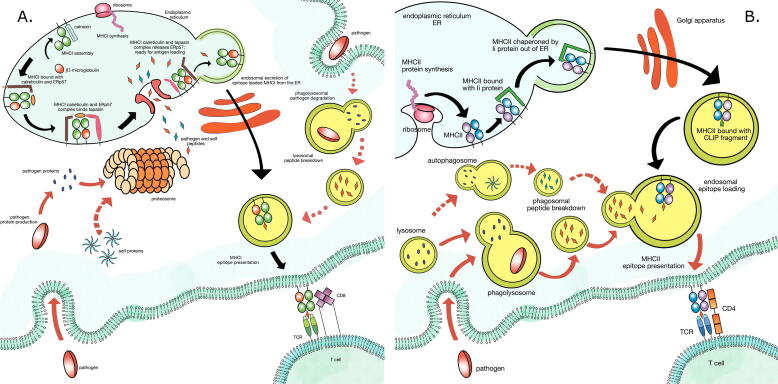
Fig. 1.
Schematic representation of MHC antigen processing and presentation adapted from cellular and immunobiological textbooks by Janeway [8] and Abbas et al. [27]. The MHC class I or II antigen presenting molecule comes into contact with CD8+ or CD4+ T cells, respectively. The binding to the T cell receptor (TCR), which induces T cell activation, is aided by the CD8 or CD4 protein, for MHC class I or II binding mechanisms, respectively. In both figures black arrows follow MHC synthesis and antigen presentation pathways. Red arrows follow antigen processing: solid - foreign-antigen direct presentation pathway; dashed - self-antigen direct presentation pathway; dotted - foreign antigen cross-presentation. A. MHC class I antigen processing and presentation. MHCI synthesis is started off by the ribosomes in the endoplasmic reticulum (ER). Additional incorporation of β2-microglobulin into the MHCI structure is aided by a transitional complex with the auxiliary protein calnexin. To protect from unsolicited interactions, the newly synthesised MHCI is complexed with calreticulin and ERp57, and subsequently to tapasin which will assist in epitope binding. Upon transporter associated with antigen processing (TAP) protein activation antigens come through into the ER and simultaneously the MHCI-tapasin-calreticulin complex releases ERp57 and widens the peptide binding cleft which allows for binding of compatible epitopes. The loaded complex is released from ER by endosome encapsulation and transported to the cell membrane to be expressed on cell surface. Foreign and self antigen processing. Some pathogens survive internalisation and continue to produce proteins in the cytosol. Alternatively, pathogens may be internalised along with their protein product. These proteins are degraded by the proteosome into peptide fragments, epitopes, and sent to the ER for peptide-MHCI assembly and presentation. Foreign epitopes are shown in orange. Self proteins follow a similar pathway of proteosomal degradation and are sent to the ER for peptide-MHC assembly and self presentation. Self epitopes are shown in blue. All nucleated cells express MHCI and follow these pathways for endogenous antigen presentation. Cross-presentation. Exogenous antigens are usually presented on MHCII expressing cells. In order to allow for MHCI presentation of exogenous antigens specialised cells process pathogens as in the MHCII pathway, but present on MHCI complexes. Several pathways might be involved in this process. The pathogen is first internalised and enzymatically degraded in the phagolysosome. The lysosome containing peptide antigens then comes into contact with synthesised MHCI molecule and form the peptide-MHCI complex. One possible pathway is that the generated antigens are transported from the lysosome, through TAP and are loaded onto the MHCI in the ER, following which they are expressed on the cell surface. Another pathway might include a vesicular loading compartment detaching from the ER, carrying the synthesised MHCI molecule, and merging with the epitope carrying lysososme. Upon merging the epitopes could load onto the MHCI and express onto the cell surface. B. MHC class II antigen processing and presentation. Pathogens are phagocytosed into the cell interior. Upon merging with a lysosome, proteases cleave the pathogen into short peptide fragments - foreign epitopes, here shown in red. The same fate befalls the cells own proteins as they undergo degradation by the autophagosome, leaving a phagosome containing short peptides - self epitopes, here shown in blue. Meanwhile, the MHCII protein is synthesised by ribosomes in the ER. Upon assembly, MHCII binds invariant chain, Ii protein. It prevents any unwanted protein binding to the MHCII complex in the ER. The Ii chaperones MHCII out of ER in an endosome. In the endosome, due to slightly acidic conditions the Ii protein degrades leaving class II associated invariant chain peptide, CLIP fragment bound in the MHCII cleft. Upon merging with a epitope containing phagosome, the MHCII comes into contact with foreign and self antigen fragments. Upon binding the peptide-MHCII complex is expressed on the cell surface where it is able to bind CD4+ T cells. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)