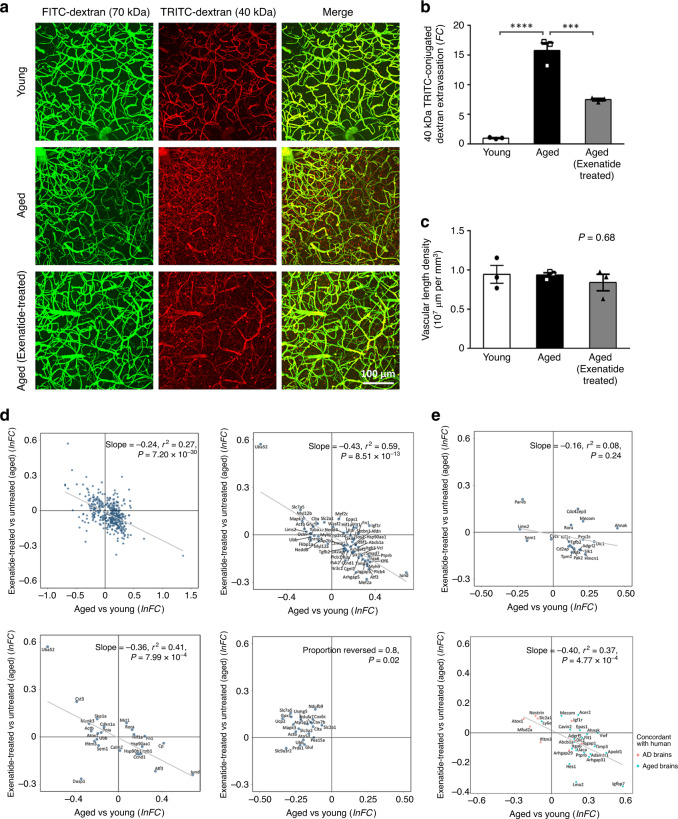
Fig. 4. Functional and transcriptomic reversal of ageing-associated endothelial changes by exenatide treatment.
a Three-dimensional rendered images (top view) of in vivo two-photon imaging of cerebral vasculature and blood–brain barrier (BBB) leakage in the mouse somatosensory cortex by co-injection of 70 kDa FITC-conjugated dextran (FITC-dextran, green) and 40 kDa TRITC-conjugated dextran (TRITC-dextran, red). FITC-dextran remained in the vasculature and allowed reconstruction of vessels, while extravasation of TRITC-dextran served as an indicator of BBB leakage which was quantified for young adult, aged and exenatide-treated aged mouse groups. b Volumetric quantification of TRITC-dextran extravasation showing BBB breakdown in aged (18–20 months old) relative to young adult mice (2–3 months old) (mean fold change (FC) in volume of extravasated TRITC-dextran relative to young adult group ± S.E.M. = 15.8 ± 1.3; P = 2.2 × 10−5 for aged vs young adult mouse group, 3 image stacks were acquired to obtain the mean for each animal, n = 3 mice for each group, one-way ANOVA with Tukey’s post-hoc test), which was significantly reduced by exenatide treatment (5 nmol/kg/day I.P. for 4–5 weeks starting at 17–18 months old, mean fold change relative to young adult group ± S.E.M. = 7.5 ± 0.2; P = 5.9 × 10−4 for exenatide-treated vs untreated aged mouse group, 3 image stacks were acquired to obtain the mean for each animal, n = 3 mice for each group, one-way ANOVA with Tukey’s post-hoc test). c, Cortical vascular length density from the three experimental groups (mean cortical vascular length density ± S.E.M. = 0.95 ± 0.11, 0.94 ± 0.03, 0.84 ± 0.11 × 107 μm per mm3 in young adult, aged and exenatide-treated aged groups respectively, P = 0.68, one-way ANOVA). Source data underlying b, c are provided as a Source Data file. d Reversal of brain capillary EC overall (left upper panel), vascular regulatory (right upper panel), immune/cytokine signaling (left lower panel) and energy metabolism (right lower panel) associated gene expression changes by exenatide treatment in aged mouse. e Reversal of aged mouse brain EC differential expressions whose human orthologs are AD GWAS genes in capEC (upper panel), or had concordant changes in human normal aged or AD brains in all ECs pooled (lower panel).