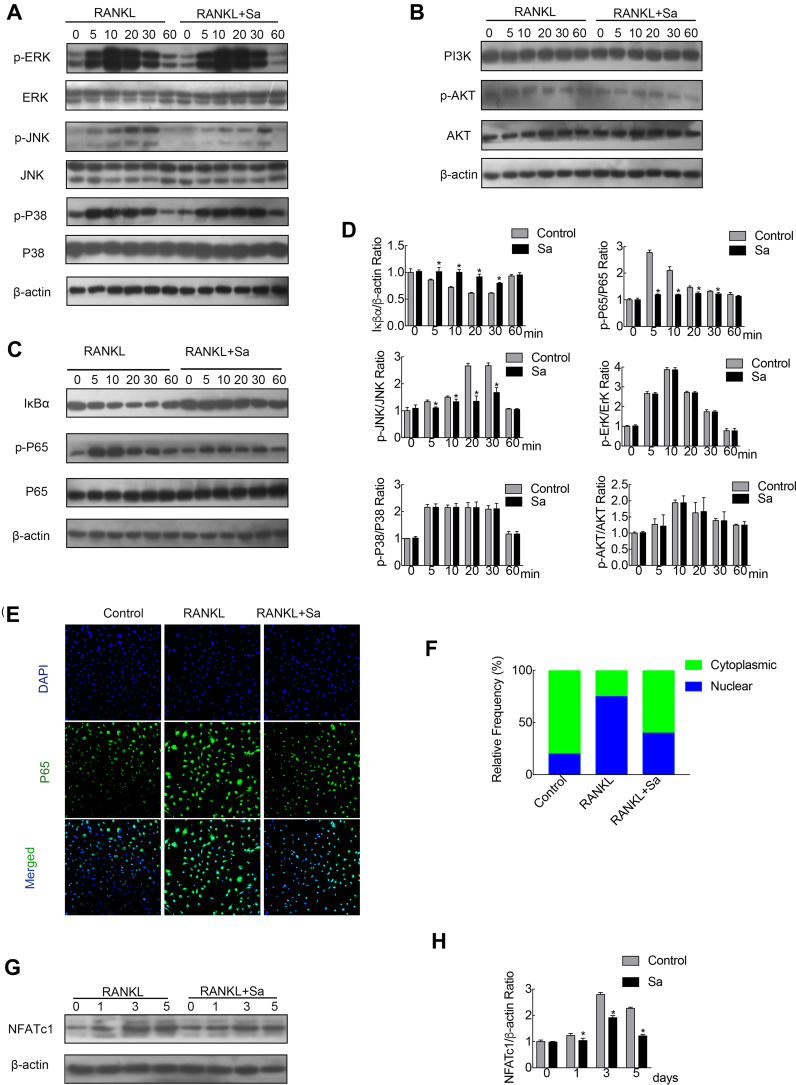
Figure 3.
Sarsasapogenin attenuates RANKL-induced activation of the NF-κB and JNK/MPAK signaling pathways. (A–C) BMMs were pretreated with 4 μM sarsasapogenin for 1 h and then stimulated with RANKL for the indicated periods. Cell lysates were probed for protein levels using Western blot analysis (n = 3). (D) Relative changes in the phosphorylation statuses of p65, Akt, ERK, p38, and JNK were determined by densitometry and expressed as a ratio against its total protein counterpart, and the expression of Iκβα relative to actin, were determined using ImageJ. (E) Sarsasapogenin prevents p65 nuclear translocation and localization. Representative immunofluorescence images of p65 localization (red) in BMMs treated with sarsasapogenin and stimulated with RANKL. Nuclei were counterstained with DAPI (blue). (F) The relative frequency of nuclear and cytoplasmic p65 under each experimental condition was quantified using ImageJ (n = 3). (G) Total cellular proteins extracted from BMM-derived osteoclasts co-treated with RANKL and 4 μM sarsasapogenin for 0, 1, 3, or 5 days were subjected to immunoblot analyses using specific antibodies to NFATc1. β-Actin was used as an internal loading control. (n = 3). Total BMM-derived osteoclasts were cultured with RANKL (100 ng/mL) and M-CSF (25 ng/mL) with or without sarsasapogenin (4 μM) for 0, 1, 3, or 5 days. Cell lysates were probed for NFATc1 protein levels using Western blot analysis (n = 3). (H) Relative expression of NFATc1 was determined by densitometry and expressed as a ratio versus actin. Bar graphs represent the mean ± SD; *p < 0.05 versus respective controls.