FIGURE 8.
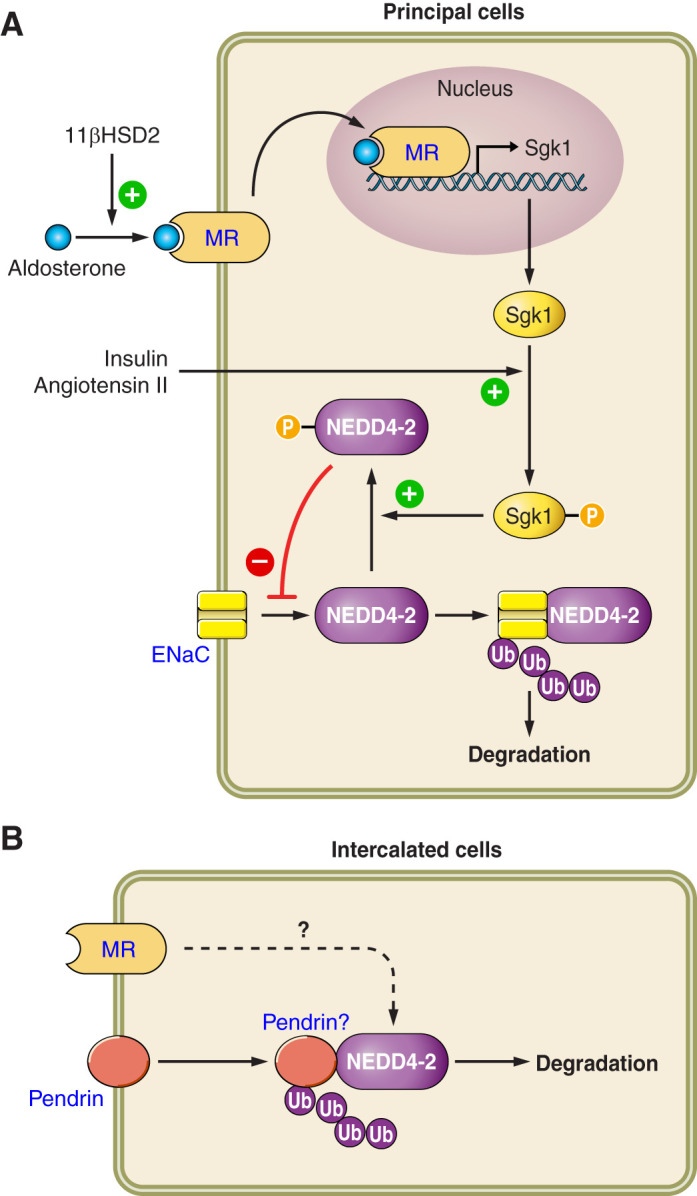
The regulation of intercalated cell (IC) and principal cell function by Nedd4-2. In principal cells, the ubiquitin ligase Nedd4-2 associates with epithelial Na+ channel (ENaC), which promotes the ubiquitylation and thus degradation of this Na+ channel. Nedd4-2 is activated through dephosphorylation, which enables it to associate with target proteins such as ENaC. Conversely, when phosphorylated, which occurs with the phosphoactivation of Sgk1 that follows insulin or angiotensin II application, Nedd4-2 cannot associate with these target proteins. When a cell expresses 11β-hydroxysteroid dehydrogenase type 2 (11βHSD2), aldosterone can bind to the mineralocorticoid receptor (MR), resulting in its translocation to the nucleus, which induces gene transcription. In ICs, Nedd4-2 regulates pendrin subcellular distribution. Whether this occurs through a direct association of Nedd4-2 and pendrin, and whether Nedd4-2 acts downstream of the MR, remains to be determined.
