Figure 4.
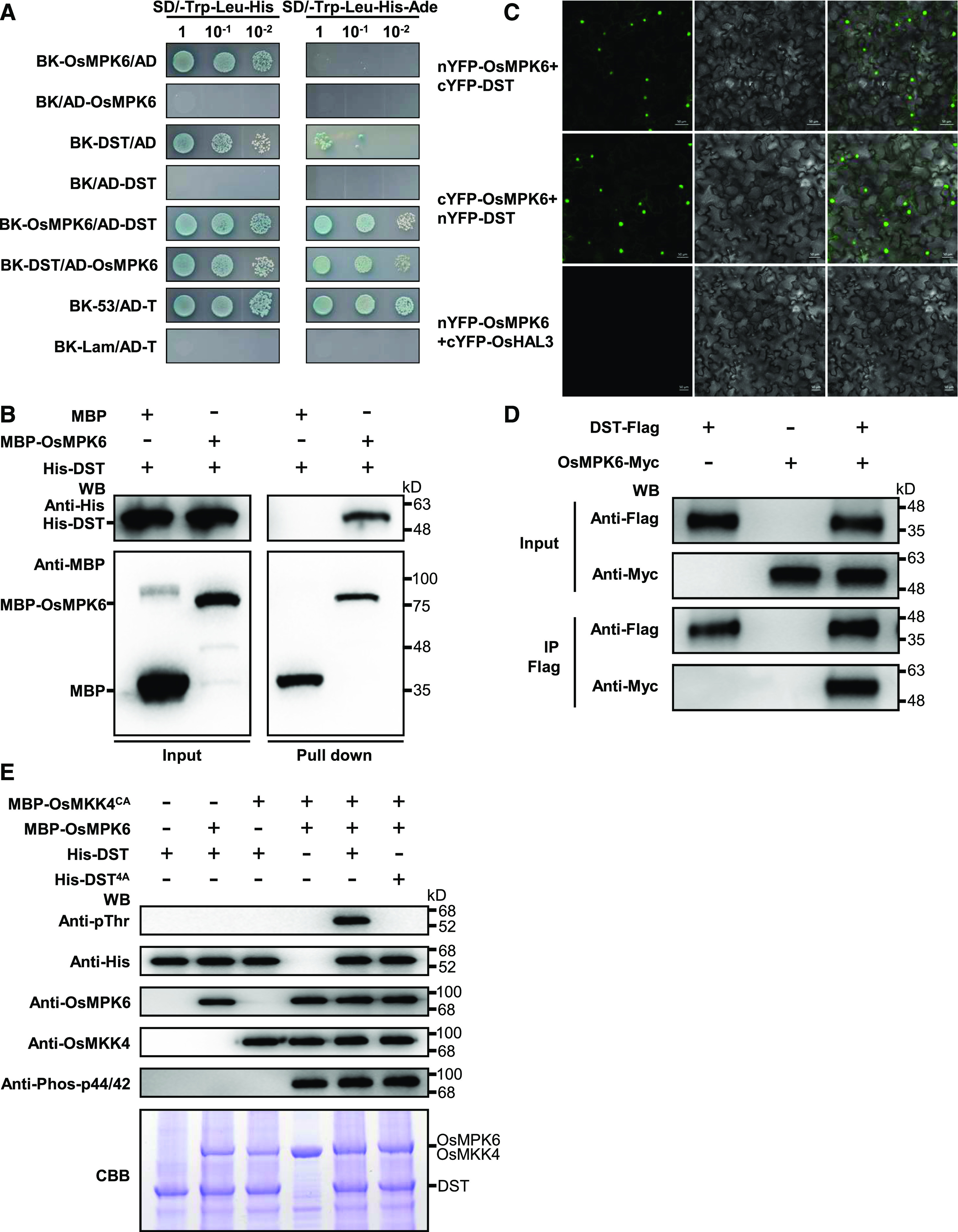
OsMPK6 Interacts with and Phosphorylates DST.
(A) Yeast two-hybrid assays indicating that OsMPK6 interacts with DST. The yeast cells were cultured on SD/-Trp-Leu-His (medium without Trp, Leu, and His) or SD/-Trp-Leu-His-Ade (medium without Trp, Leu, His, and Ade) containing X-ɑ-gal. BK (pGBKT7) and AD (pGADT7) are the bait and prey vectors, respectively.
(B) In vitro pull-down assays of His-tagged DST using MBP-tagged OsMPK6. Pull-down was verified by immunoblotting with anti-His and anti-MBP antibodies. WB, Western Blot.
(C) OsMPK6 associates with DST, as shown by BiFC assays in N. benthamiana leaf cells. OsMPK6 and DST were both fused to the N-terminal fragment of YFP (nYFP) and the C-terminal fragment of YFP (cYFP). nYFP-OsMPK6 and cYFP-DST or cYFP-OsMPK6 and nYFP-DST were then co-expressed in N. benthamiana leaves. The nYFP-OsMPK6 and cYFP-OsHAL3 fusion proteins were used as negative controls.
(D) Co-IP assays indicating that OsMPK6 interacts with DST in planta. Pro35S:DST-Flag and Pro35S:OsMPK6-Myc fusions were co-expressed in N. benthamiana leaves. Proteins were extracted (Input) and immunoprecipitated (IP) with Flag beads. The immunoblot assays were performed using anti-Flag and anti-Myc antibodies. WB, Western Blot.
(E) OsMPK6 phosphorylates DST in vitro. The MBP-OsMKK4CA, MBP-OsMPK6, His-DST, and His-DST4A fusion proteins were expressed in E. coli and purified. The in vitro phosphorylation reactions were performed using the purified proteins. DST phosphorylation was detected with the anti-Phospho-Thr antibody. Recombinant OsMKK4, OsMPK6, and DST were detected using anti-OsMKK4, anti-OsMPK6, and anti-His, respectively. Phosphorylated OsMPK6 was detected with anti-Phospho-p44/42. The gel stained with Coomassie brilliant blue (CBB) was used as a loading control. WB, Western Blot.
