Figure 1.
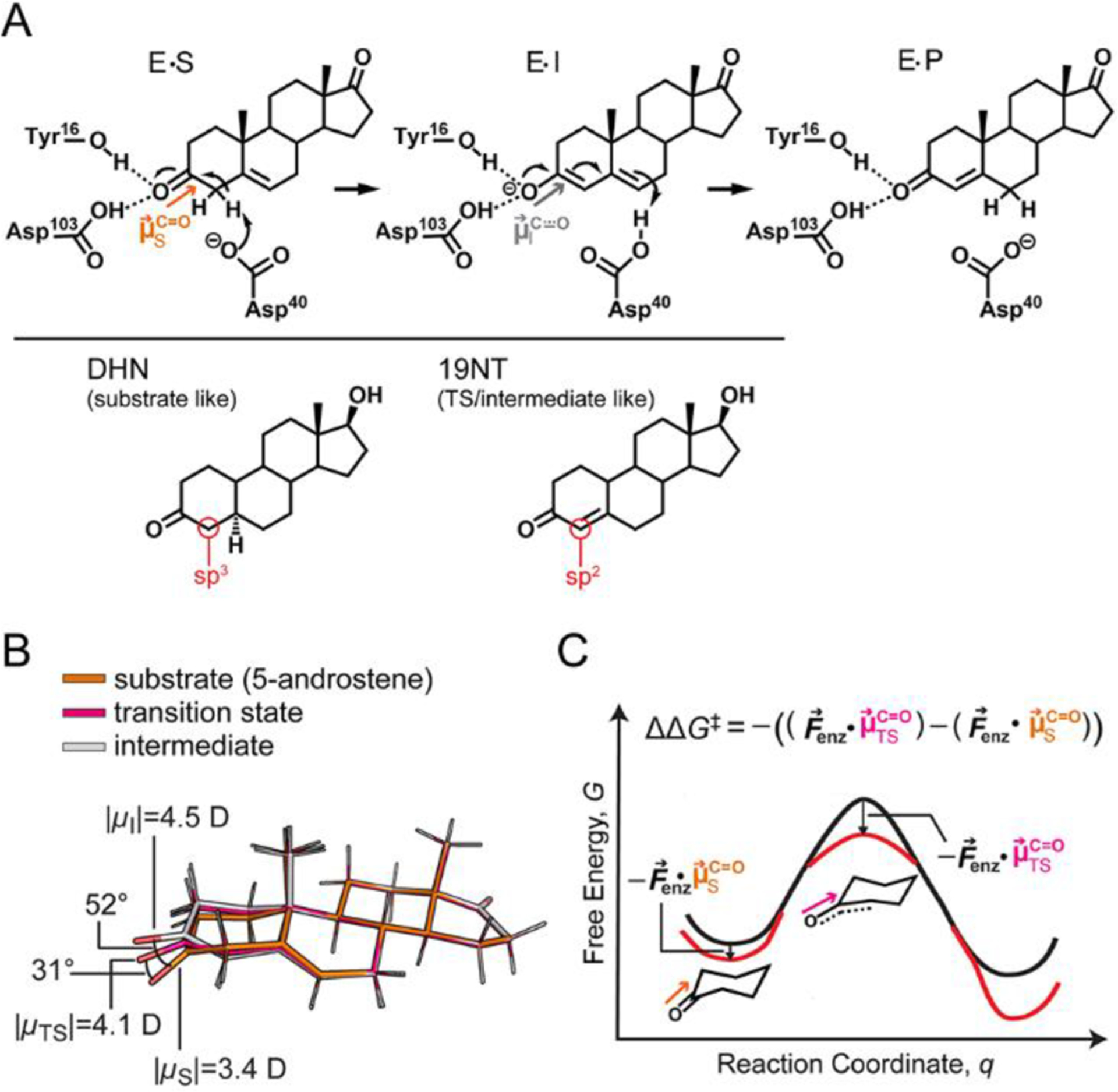
Electrostatic stabilization in the context of ketosteroid isomerase (KSI). (A) KSI catalyzes the isomerization of 5-androstenedione to 4-androtenedione via a dienolate intermediate. The negative charge on the oxygen in the transition state (TS) and intermediate state (I) is stabilized by the oxyanion hole. As the reaction proceeds from the GS to the TS and I, the hybridization of the C4 carbon (red circle) of the substrate changes from sp3 to sp2. 5α-dihydronandrolone (DHN) and 19-nortestosterone (19NT) are used to mimic the two states, respectively. (B) Ab initio calculations on the substrate, TS, and I of KSI’s reaction in the gas phase suggests electrostatic and geometric changes occur along the reaction coordinate. (C) The electric field of KSI, , preferentially stabilizes the TS because of its larger bond dipole. Furthermore, the angle change shown in part B could result in the C−O dipole in the TS becoming better aligned with leading to additional stabilization. Equation 1 encompasses both the scaling effect and orientational effect of electrostatic catalysis.
