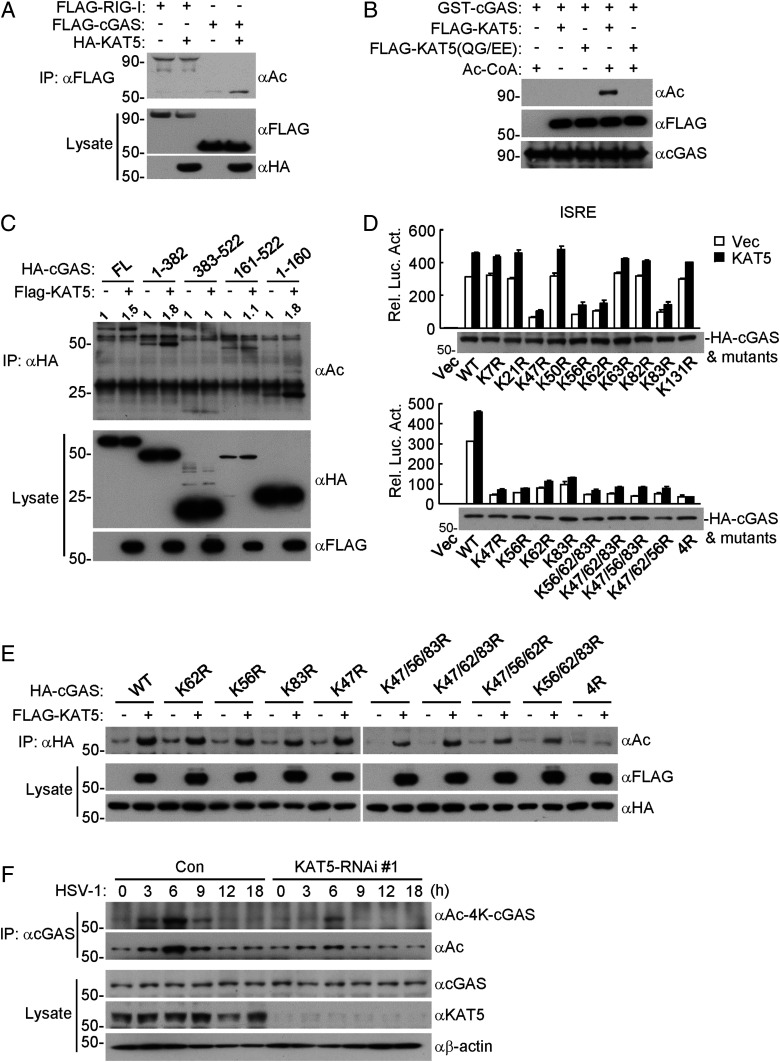
Fig. 3.
KAT5 mediates acetylation of the N-terminal domain of cGAS. (A) Overexpression of KAT5 increased acetylation of cGAS but not RIG-I. HEK293 cells were transfected with the indicated plasmids for 20 h, and then the cells were treated with the deacetylase inhibitors TSA and NAM for 8 h before coimmunoprecipitation and immunoblotting analysis were performed with the indicated antibodies. (B) Effects of KAT5 and its mutant on acetylation of cGAS in vitro. GST-cGAS was purified from E. coli. FLAG-KAT5 and FLAG-KAT5(QG/EE) were purified from HEK293. The indicated purified proteins were incubated for 2 h, followed by immunoblotting analysis with the indicated antibodies. (C) Effects of KAT5 on acetylation of cGAS and its truncated mutants. The experiments were similarly performed as in A. (D) Effects of KAT5 on ISRE activation mediated by cGAS and its mutants. The HEK293 cells stably expressing MITA were transfected with the indicated plasmids for 24 h before luciferase assays were performed. (E) Effects of KAT5 on acetylation of cGAS and its N-terminal lysine mutants. The experiments were similarly performed as in A. (F) Effects of KAT5 knockdown on HSV-1–induced acetylation of cGAS. The KAT5-knockdown or control THP-1 cell lines were infected with HSV-1 (MOI = 2) for the indicated times before coimmunoprecipitation and immunoblotting analysis were performed with the indicated antibodies.