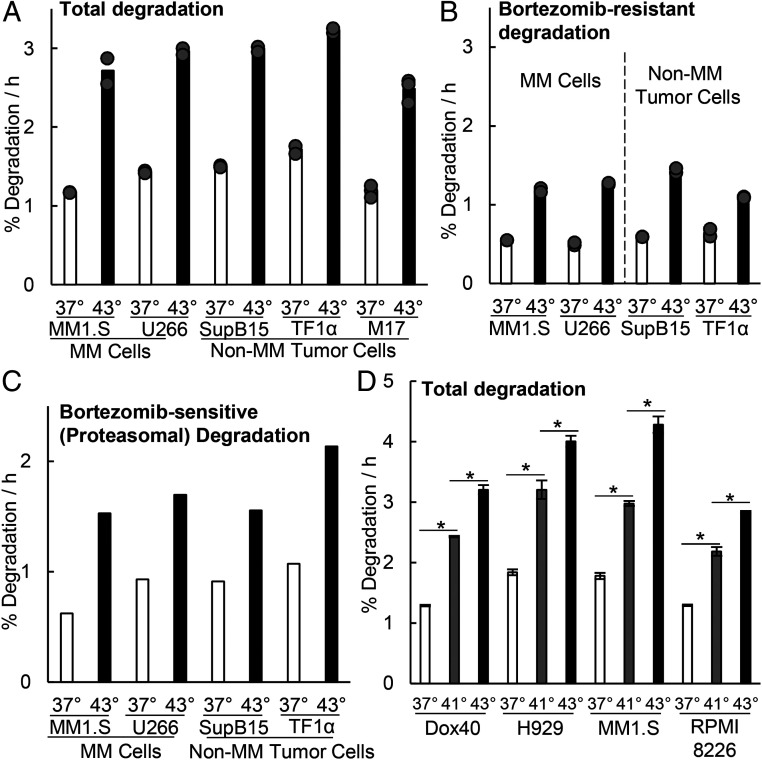
Fig. 2.
Exposure of myeloma cells to 43 °C increases degradation of long-lived cellular proteins by the proteasome. After switching to 43 °C, the myeloma and nonmyeloma cells tested all increased their overall rates of protein degradation and proteasome-mediated degradation by about twofold. (A and B) Long-lived cell proteins (the bulk of cell proteins) were labeled with 3H-phenylalanine at 37 °C, and after a 2 h chase to allow clearance of short-lived proteins, (A) their degradation rates were measured for 2 h (31, 62) when cells were exposed to 43 °C or kept at 37 °C, and (B) the BTZ-resistant degradation represents the rates of proteolysis measured in these cells in the presence of 1 µM BTZ and reflects primarily lysosomal degradation (31, 62). Data shown are from three biological replicates for M17 cells and two for other cells. (C) Proteasomal degradation (i.e., BTZ-sensitive) rates also increased about twofold at 43 °C. These rates were calculated by subtracting the average of the BTZ-resistant degradation rate (B) from the average total degradation rate (A) as described previously (31, 62). The BTZ-inhibited fraction composed about 60% of the proteolysis at both 37 and 43 °C. (D) At 41 °C, overall rates of protein degradation also increased but by less than at 43 °C. Degradation rates were measured in four myeloma lines exposed for 2 h to 41 or 43 °C or maintained at 37 °C. Error bars are SDs (n = 4). *P < 0.05.