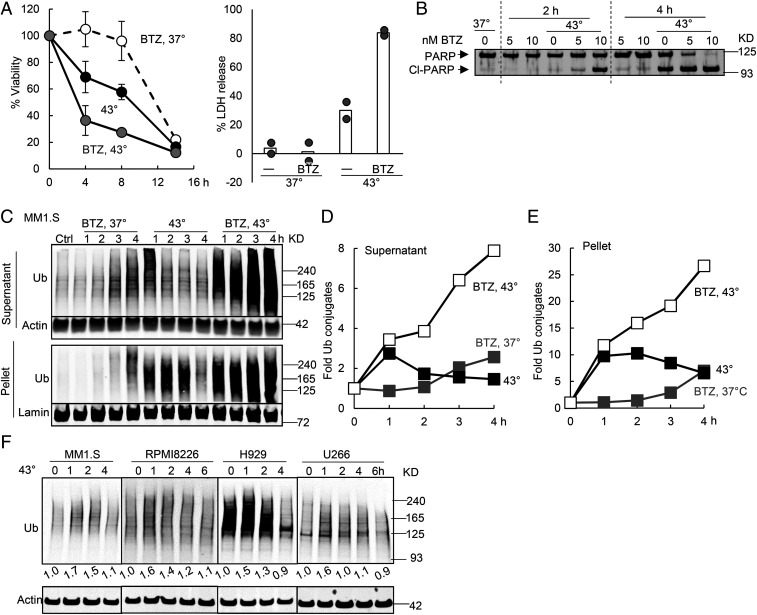
Fig. 3.
Inhibiting the proteasome during the exposure of myeloma cells to 43 °C caused synergistic buildup of ubiquitinated proteins and apoptosis. (A) MM1.S cells were incubated at 37 or 43 °C with or without 10 nM BTZ. Exposure to BTZ at 43 °C caused synergistic killing when cell viability was measured by the MTS assay (Left, n = 3, error bars are SDs) or LDH release (Right, n = 2, both data points for two typical experiments are shown). (B) Exposure of MM1.S cells to 10 nM BTZ at 43 °C caused PARP cleavage by 2 h, but exposure to BTZ or 43 °C alone for 2 h did not. (C–E) Exposure of MM1.S cells to 43 °C caused a rapid accumulation of ubiquitinated proteins (detected by K48 linkage-specific antibody) especially in the 10,000 × g pellet. Their levels then decreased in both the supernatant and the pellet due to proteasomal degradation since BTZ prevented this decrease. Cells were exposed to 43 °C with or without 10 nM BTZ. Cell proteins were fractionated by centrifugation at 10,000 × g for 10 min in the presence of 1% Triton X-100 and analyzed by Western blotting (C). The amount of ubiquitinated proteins in C was quantified in the supernatant after normalization to actin (D) or in the pellet after normalization to lamin (E). (F) When four myeloma lines were shifted to 43 °C, there was a rapid accumulation of ubiquitin conjugates (detected with P4D1 antiubiquitin antibody) in 1 h, followed by a decrease.