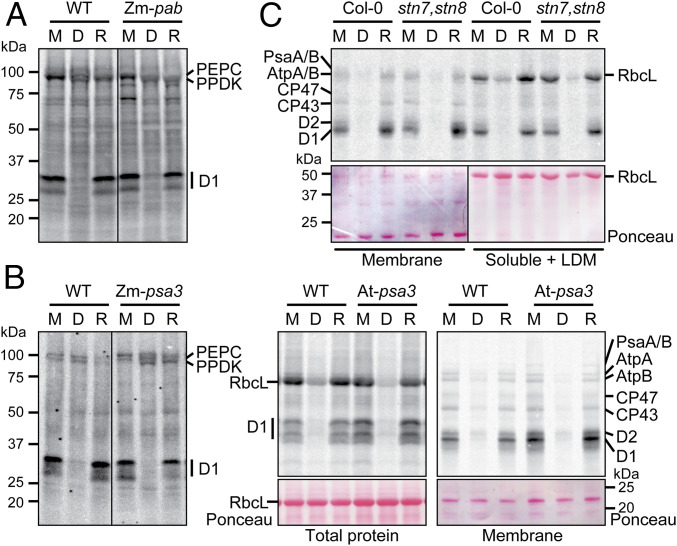
Fig. 3.
Analysis of light-regulated D1 synthesis in mutants lacking ATP synthase, PSI, or the STN7 and STN8 kinases. Plants were pulse labeled at midday (M), after 1 h in the dark (D), or after a 15-min reillumination (R). Maize mutants (Zm-pab and Zm-psa3) were radiolabeled for 15 min in the absence of cycloheximide, and total leaf lysates were analyzed by SDS/PAGE with sample loading normalized based on labeling of two cytosolic proteins at ∼100 kDa (presumed to be PEPC and PPDK). Arabidopsis mutants (At-psa3 and stn7,stn8) were radiolabeled for 20 min in the presence of cycloheximide, and samples were loaded on the basis of equal chlorophyll. (A) Analysis of a maize Zm-pab mutant, lacking the thylakoid ATP synthase. The mutant is described in SI Appendix, Fig. S1A. Total leaf lysates are shown. The line separates noncontiguous lanes from the same exposure of the same gel. (B) Analysis of mutants lacking PSI in maize (Zm-psa3) and Arabidopsis (At-psa3). Arabidopsis samples were analyzed both as total leaf lysates (Left) and as membrane fractions (Right). The nitrocellulose filters harboring the radiolabeled proteins were stained with Ponceau S to illustrate equal sample loading. RbcL, large subunit of Rubisco. The line in the Zm-psa3 panel separates noncontiguous lanes from the same exposure of the same gel. (C) Analysis of an Arabidopsis stn7,stn8 double mutant. The experiment was performed as for At-psa3 except that radiolabeled RbcL was visualized by analysis of supernatants remaining after membranes were pelleted by centrifugation. This supernatant is designated soluble + LDM because it includes low-density membranes (LDMs), which harbor some of the radiolabeled D1. See SI Appendix, Materials and Methods for details. A single Ponceau S-stained blot was imaged with different contrast (marked by a line) to improve visibility of the bands.