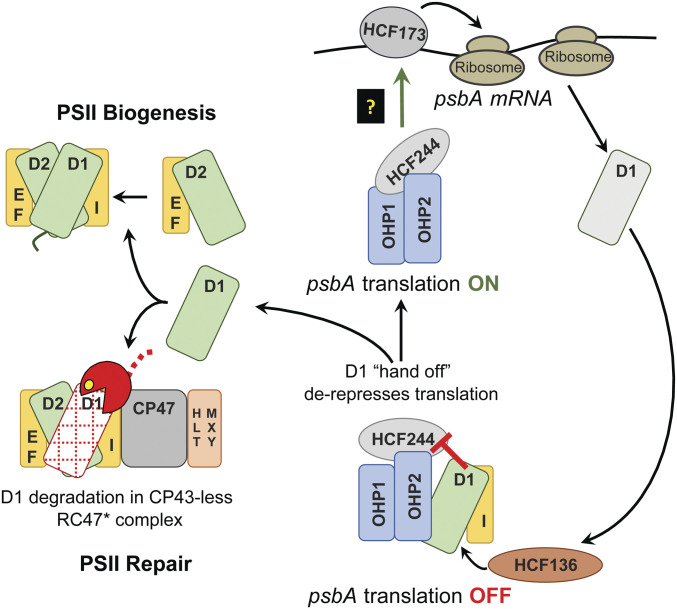
Fig. 6.
Model for the coupling of psbA translation to light-induced D1 damage. The HCF244/OHP1/OHP2 complex is diagrammed at Bottom with bound D1 and PsbI. This form of the complex is referred to as the D1 module in Synechocystis (63). OHP1 and OHP2 are integral thylakoid proteins, whereas HCF244 is bound to the stromal face of the membrane (32, 34, 35, 63). Current data suggest that HCF136 (on the luminal face of the membrane) is required to insert nascent D1 into the complex, possibly in conjunction with the insertion of chlorophyll (29–31, 64). We posit that the presence of D1 in this complex inhibits the ability of the stroma-exposed components (HCF244 and OHP2) to activate psbA translation. Repression is relieved when D1 is transferred out of the complex, either to a repair intermediate from which damaged D1 had been removed (Bottom) or to the “D2 module” during PSII biogenesis (Top). We show the canonical RC47* complex (6, 7) as the repair intermediate that accepts nascent D1, but the mechanism would be similar with the alternative repair intermediate detected recently in Synechocystis (77). We propose that the D1-less HCF244 complex communicates with HCF173 in the stroma, which is known to bind the psbA 5′ UTR and to activate translation initiation (48, 79, 81).