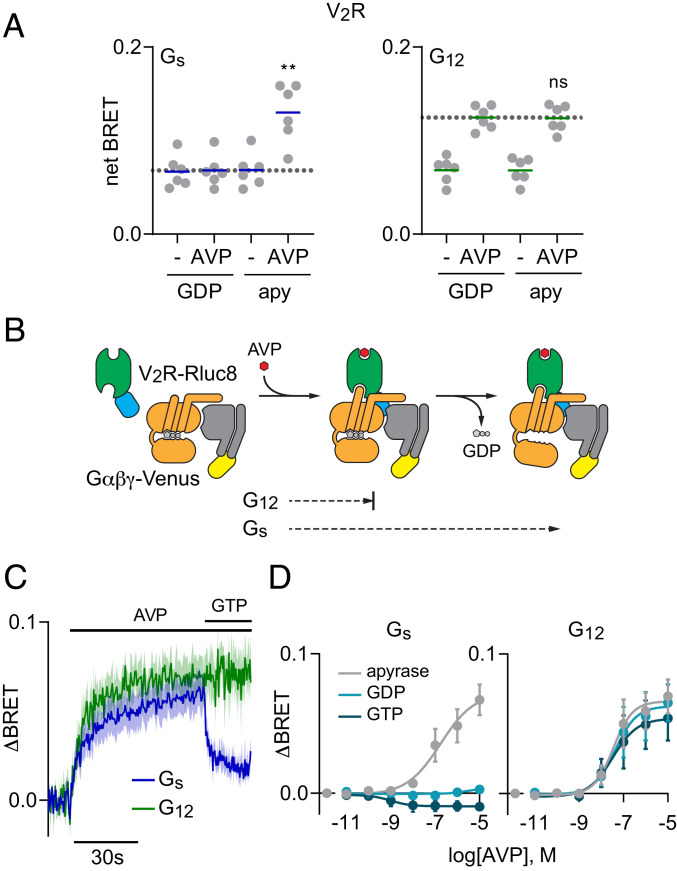
Fig. 1.
V2R forms GDP-resistant agonist-induced complexes with G12 heterotrimers. (A) BRET between V2R-Rluc8 and Gαβγ-Venus in the presence or absence of AVP (1 μM), and the presence or absence of GDP. When GDP was absent, apyrase (apy) was added to remove residual nucleotides. AVP-induced BRET to Gs (Left) but not G12 (Right) heterotrimers was enhanced when GDP was absent; **P < 0.005; n.s., not significant (P = 0.58); one-way ANOVA (Sidak’s test) compared to GDP+AVP; n = 6. (B) Cartoon representation of two steps of V2R-G protein coupling: agonist-induced formation of receptor-G protein complexes, and GDP release. (C) Time course of BRET between V2R-Rluc8 and Gαβγ-Venus in response to injection of 1 μM AVP, followed by injection of 100 μM GTP in permeabilized cells treated with apyrase (mean ± SEM; n = 4–6). (D) BRET between V2R-Rluc8 and Gαβγ-Venus as a function of AVP concentration in permeabilized cells expressing either Gs (Left) or G12 (Right) heterotrimers treated with apyrase, GDP (100 μM), or GTP (100 μM). The logEC50 for association with Gs was −6.8 ± 0.5 in apyrase-treated cells, and the logEC50s for association with G12 were −7.5 ± 0.3, −7.4 ± 0.4, and −7.5 ± 0.5 in the presence of apyrase, GDP, and GTP, respectively. Data points represent the change in BRET (ΔBRET) in response to AVP (mean ± SEM; n = 3).