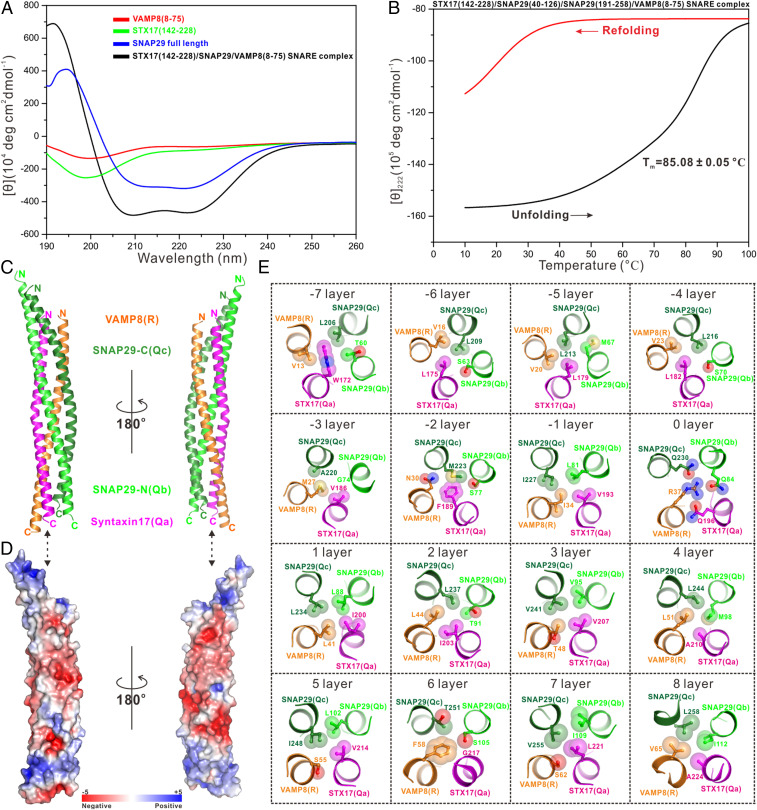
Fig. 5.
Biochemical and structural characterizations of the STX17–SNAP29–VAMP8 SNARE complex. (A) Overlay plot of the CD spectra of the STX17–SNAP29–VAMP8 SNARE complex and related individual autophagic SNARE proteins. (B) Thermal unfolding and refolding analyses of the STX17–SNAP29–VAMP8 SNARE complex monitored by CD spectroscopy at 222 nm. (C) Ribbon diagram showing the overall structure of the STX17–SNAP29–VAMP8 SNARE complex. (D) Surface charge potential representation (contoured at ±5 kT/eV; blue/red) of the STX17–SNAP29–VAMP8 SNARE complex with the same orientation as in C, revealing two highly negatively charged patches. (E) Detailed interior interactions of the autophagic STX17–SNAP29–VAMP8 SNARE complex formed by 16 layers of interacting amino acid side chains.