Abstract
Purpose of the Review
Reverse shoulder arthroplasty (RSA) is commonly considered as one of the options for surgical management of the functionally irreparable rotator cuff tear (FIRCT). This article reviews tips and tricks to optimize the outcome of RSA when performed specifically for this indication.
Recent Findings
RSA has been reported to provide satisfactory outcomes in a large proportion of patients with FIRCTs. However, subjective satisfaction is lesser in patients with well-maintained preoperative motion as well as those with isolated loss of active external rotation. The popularity of implants that provide some degree of global lateralization continues to increase. Optimizing the outcome of RSA for FIRCTs requires a careful balance between minimizing perimeter impingement and enhancing the function of intact muscles, in particular the deltoid and any remaining rotator cuff. Controversy continues regarding the benefits and disadvantages of subscapularis repair at the time of RSA. Tendon and muscle transfers performed at the time of RSA have the potential to optimize the outcome in selected patients with profound weakness in external rotation or those with severe deltoid dysfunction.
Summary
When RSA is considered for patients with a FIRCT without arthritis, careful attention to indications and technical pearls may contribute to optimize outcomes.
Keywords: Rotator cuff repair, Reverse shoulder arthroplasty, Cuff tear arthropathy, Tendon transfers, Latissimus dorsi, Massive irreparable cuff tear
Introduction
Reverse shoulder arthroplasty (RSA) was designed to improve the outcome of shoulder replacement in the setting of rotator cuff insufficiency [1••]. Although cuff-tear arthropathy was the main condition managed with RSA initially [2], indications for this procedure expanded to other disorders, including the functionally irreparable rotator cuff tear (FIRCT) without arthropathy [3]. Many people around the world with FIRCTs have benefited from RSA. However, a proportion of patients who undergo RSA specifically for FIRCT do not do as well [4••]. In addition, RSA comes with some limitations—namely unpredictable restoration of axial rotation—and potential complications, including dislocation and postoperative fractures of the scapular acromion or spine [5].
Over the last two decades, the designs of RSA implants have been modified and expanded substantially, and many of the prosthesis available today vary widely [6••]. In parallel, a number of salvage procedures have been developed or reinvigorated for the management of FIRCTs, including patch augmentation, superior capsular reconstruction (SCR), implantation of an absorbable balloon, and contemporary tendon transfers [7]. Obtaining the best possible outcome when RSA is performed for subjects with FIRCTs requires selecting the correct patients for the procedure, choosing the best possible implant configuration, and implanting it according to plan, understanding how to obtain the ideal soft-tissue balance and tension for each shoulder and consider adjuncts such as tendon transfers when needed.
When to Consider RSA for FIRCTs without Arthropathy
The relative indications of various salvage procedures for the surgical management of FIRCTs remain controversial [7]. However, there are a number of factors that may incline the balance in favor of reverse (Table 1). Static anterior subluxation is considered by many as a contraindication for any of the alternative salvage procedures, except maybe an anterior latissimus dorsi transfer if mid-term follow-up studies end up proving its efficacy. A pan-circumferential irreparable cuff tear (involving the subscapularis, supraspinatus, and infraspinatus, with or without extension into the teres minor) could be salvaged combining two procedures (two tendon transfers, or anterior tendon transfer plus SCR), but most would favor RSA. There is controversy regarding the efficacy of joint-preserving procedures for reversing pseudoparalysis, but most would agree that RSA is most predictable in that regard, especially in the presence of anterosuperior escape. RSA is also favored for older patients with advanced age and lower physical demands. Finally, RSA is considered when alternative salvage procedures have failed, for cuff failure complicating anatomic shoulder arthroplasty, as well as when the majority of the pain is suspected to be secondary to articular cartilage degeneration and arthritis.
Table 1.
Factors that would incline the balance in favor of reverse shoulder arthroplasty versus other salvage procedures
|
• Static anterior subluxation • Combined irreparable subscapularis + posterosuperior cuff tear • Anterosuperior scape • Advanced age/lower demand • Failed prior salvage procedure • Cuff failure complicating anatomic shoulder arthroplasty • Glenohumeral joint considered major reason for pain |
Some patients with a FIRCT are particularly at risk for a poor outcome or subjective dissatisfaction after RSA. Patients with preserved active motion in all directions may be disappointed if their motion decreases after RSA [4••], particularly if their pain level is mild to moderate. Anecdotally, we have found that patients are less satisfied after RSA when their preoperative subjective shoulder value (SSV) [8] is relatively high. A second category of patients that may not be as satisfied after RSA are those with isolated loss of active external rotation [9]. Even though improved external rotation has been reported after RSA in patients with an intact teres minor, especially with use of implants that provide a larger lateral offset [10], patients with preserved active elevation but pseudoparalysis in external rotation are better candidates for a tendon transfer. In our practice, a lower trapezius transfer is preferred to a latissimus dorsi transfer to restore active external rotation in shoulders of patients with isolated loss of active external rotation [11]. Finally, patients with compromised deltoid function secondary to a nerve injury or prior surgery may not benefit from RSA unless the deltoid dysfunction is addressed as well [12, 13].
Two Basic Concepts: Perimeter Impingement and Soft Tissue Tension
When an RSA is implanted, preoperative planning and procedural execution must consider two important aspects: perimeter impingement and soft tissue tension.
Perimeter Impingement
After implantation of an RSA, there is the potential for impingement between the humerus and scapula and also between the humeral implant and the scapula. The most widely recognized consequence of perimeter impingement after RSA is scapular notching [14, 15]. Notching is the impingement of the inferomedial aspect of the humeral polyethylene component with the lateral scapular column leading to polyethylene wear, scapular bone resorption, and osteolysis. Table 2 summarizes common sources of impingement. Some degree of impingement will occur at the very extreme ends of the motion arcs; the goal is to minimize perimeter impingement within the expected functional motion arcs.
Table 2.
Perimeter impingement locations
|
• Inferomedial polyethylene with lateral scapular pillar ▪ Mostly in axial rotation and extension ▪ Results in notching, polyethylene wear, humeral osteolysis • Lesser tuberosity with coracoid and conjoined tendon ▪ Mostly in cross-body adduction ▪ Results in anterior shoulder pain and limited cross-body adduction • Greater tuberosity with acromion and spine of the scapula ▪ Mostly in abduction and elevation ▪ Results in limited abduction and elevation, possibly involved in stress fractures of the acromion and spine |
Conceptually, impingement can be minimized if (1) the humerus and humeral component land in space away from the scapula in an inferior, lateral, and posterior direction and (2) all osteophytes creating the potential for impingement are removed; it may require trimming some bone from the lesser or greater tuberosity. Multiple factors can contribute to minimizing perimeter impingement: a varus polyethylene opening angle, glenoid lateralization, posteroinferior glenoid overhang, adequate inferior inclination, larger glenosphere, and others [14, 16]. Preoperative planning and intraoperative assessment and correction of impingement are extremely helpful to minimize perimeter impingement. However, it must be understood that many adjustable parameters to minimize perimeter impingement in one plane, may increase it in another. For example, a varus polyethylene opening angle will decrease inferior impingement against the lateral column of the scapula; however, it may also increase impingement of the greater tuberosity against the acromion laterally. Overall, a hierarchy of parameters exists, which is likely surgeon and patient specific, and in the end, a compromise must be met to optimize the most important motions.
Soft Tissue Tension
Implantation of an RSA also leads to a change in the tension of several musculotendinous units around the shoulder. Depending on the design and sizing selected—and the position where the surgeon secures implants to bone—the impact of RSA on soft tissue tension will vary. Deltoid tension seems to increase universally with all implants currently available. The effect of RSA on any intact rotator cuff or transferred tendons is not completely understood and varies more as a function of the design selected and the implantation technique.
Implanting an RSA in a cuff-deficient shoulder with the ideal soft tissue tension for each particular patient remains an art and to some extent, elusive. Insufficient soft tissue tension may contribute to prosthesis dislocation or weakness with poor active motion. Excessive soft tissue tension may lead to stretch neuropathy, pain from overstretched muscles, acromial or spine stress fractures, and poor cosmetic appearance of the shoulder.
Tension influences muscle force generation. There is a relation between muscle length and force generation, summarized in its simplest form by Blix principles: active force generation increases as sarcomere myofilament overlap increases (optimal human sarcomere length 2.6–2.8 μm), and passive force generation increases proportionally to muscle length, which translates into longer sarcomeres and longer extracellular tissues. Interestingly, some muscles exhibit relatively high passive tension at short lengths, whereas others require much more stretch to exhibit passive tension [17]. Excessive muscle lengthening will lead to lack of myofilament overlap, which would decrease active force generation.
However, muscle length is only one of the factors that determines the true power of each single muscle; moment arm, line of action or pull, fiber composition, and cross-sectional area all influence power generation. In addition, for abnormal shoulders undergoing arthroplasty, the force generated by a given muscle may be influenced by the integrity and power of potential antagonists, as well as the degree of atrophy and fatty infiltration of the muscle being assessed.
These principles are directly related to optimization of muscle function or force after RSA implantation. For example, with a Grammont style prosthesis, some have proposed that deltoid force generation is optimized by changes in moment arm, line of action, and length; this same implant configuration may lead to slacking of any remaining posterior rotator cuff, which could translate in loss of strength in external rotation. Our goal as surgeons should be to select an implant and position it in the shoulder so that the force of each relevant muscle is optimized. Unfortunately, accurate information on what combination of length, moment arm, and line of action is ideal for each of the shoulder muscles is lacking.
A Potential Conflict
In certain shoulders, it may be possible to both minimize perimeter impingement and optimize soft tissue tension. However, in many shoulders, a compromise is necessary: the degree of humeral translation in space needed to minimize perimeter impingement may exceed the ability of the remaining soft tissues (namely, muscles and neurovascular structures) to function properly, and it may also translate in excessive stress on bone. RSA should be specifically planned for each patient considering the patient’s size, which specific muscles are deficient, the condition of remaining intact muscles, bone quality, and scapular kinematics, among other factors. When critical rotator muscles are deficient, tendon transfers can help optimize the outcome after RSA.
Prosthesis Selection and Implantation
Publications and presentations about RSA continue to use the terms medialized and lateralized implant when classifying prostheses [18]. Unfortunately, such oversimplification is obsolete in a time when there is wide variation of implant designs [6••]. It is also important to emphasize that the final biomechanics of RSA will depend not only on the implant selected but also on how the prosthesis was implanted. For example, varus or valgus malalignment, particularly common with certain short stems, will have a major influence on the final polyethylene opening angle [19]. The same applies to how much proximal humerus is resected, where the baseplate is implanted in terms of height and superoinferior inclination and several other factors. In this review article, we provide our current philosophy of prosthesis selection and implantation when RSA is performed for FIRCT (Table 3).
Table 3.
Authors’ preferred guidelines for RSA implantation in the cuff-deficient shoulder (FIRCT)
| Glenoid component |
• Posteroinferior glenosphere overhang (2–5 mm) • Lateralization (2–10 mm) ▪ Built into glenosphere ▪ Built into baseplate ▪ With bone (BIO-RSA) • Larger size glenospheres • Neutral or anatomic version • Neutral inclination as referenced to supraspinatus fossa (inferior tilt as referenced to the face of the glenoid) |
| Humeral component |
• 135 or 145-degree polyethylene opening angle • Onlay vs inlay depends on patient/implant design |
| Soft tissue tension |
• Ease of trial reduction and dislocation • Tension on deltoid and conjoined tendon |
| Subscapularis management | • Repaired when possible |
| Lat dorsi ± teres major transfer |
• Very limited active external rotation • Positive Hornblower’s sign • Severe teres minor fatty infiltration |
Preoperative Planning and Assisted Operative Execution
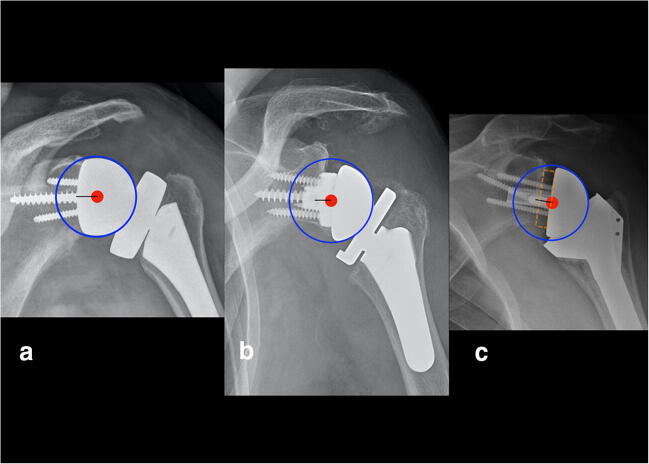
The authors believe that preoperative planning of RSA implantation using software packages provides tremendous value to understand how to best minimize perimeter impingement and optimize component position, including correction of bone deformity and ideal component sizing and fixation (Fig. 1) [20]. The main piece of information lacking in currently available planning software packages relates to prediction and optimization of soft tissue tension. Assisted execution and intraoperative adaptation of the preoperative plan will take us one step further along the optimization road. Assistance may come in the form of patient-specific instrumentation, mixed reality, navigation, and robotic-assisted surgery. The relative value of these technologies hinges on their quality and cost.
Fig. 1.
Preoperative planning software helps minimize perimeter impingement and optimize component position. The red sphere identifies impingement between the medial polyethylene liner and the lateral scapular pillar
Glenoid Component
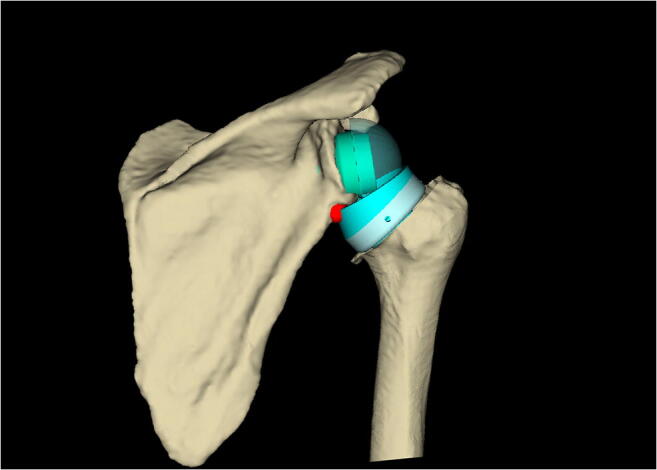
Use of a relatively larger glenosphere with some posteroinferior overhang in reference to the inferior glenoid rim helps minimize perimeter impingement. Some degree of glenoid lateralization is also beneficial; lateralization may be built in the glenosphere or baseplate, or it can be achieved by grafting under the baseplate (so-called Bony increased offset-RSA or BIO-RSA [21] (Fig. 2). The optimal amounts of inferior overhang and lateralization are not agreed upon, but they are probably in the range of 2–5 mm and 2–10 mm, respectively. The position of the baseplate on the bony glenoid surface should be primarily determined by the desired position of the glenosphere, which most of the times translates into placement on the inferior aspect of the glenoid face. For the purposes of this article, we will not consider patterns of bone erosion in cuff tear arthropathy, since we are reviewing optimization of RSA for the FIRCT without arthropathy. As such, we would be dealing with no acquired glenoid bone loss (Favard E0 glenoids) [22]. In such circumstances, in terms of version, we believe it is acceptable to (a) position the baseplate perpendicular to Friedman’s line or (b) respect native retroversion (to minimize reaming) as long as it is under 10° [23]. For inferior inclination, we aim to position the component perpendicular to the floor of the supraspinatus fossa [24].
Fig. 2.
Glenoid lateralization may be achieved through the glenosphere (a), baseplate (b) or using bone graft (c)
Humeral Component
A polyethylene opening angle of 135° minimizes perimeter impingement inferiorly [25••]. A major area of controversy relates to the use of inlay vs onlay prosthesis. Theoretical benefits of an inlay humeral component include (1) closer location of the pivot point to the geometric center of the proximal humerus and (2) less risk of excessive soft tissue tension when combined with glenoid lateralization. Benefits of onlay humeral components include (1) preservation of proximal humerus bone stock, (2) easier convertibility, and (3) more adequate overall soft tissue tension when combined with glenoid medialization. The authors currently use an onlay humeral component, but when substantial glenoid lateralization is preferred, inlay components or ultra-thin humeral bearings maybe preferred. True inlay or onlay behavior is particularly susceptible to surgical technique: larger inlay components may not completely fit in smaller metaphyses, thus behaving as onlay; by the same token, when the posterosuperior cuff is deficient, the surgeon may resect more proximal humerus when implanting an onlay component to decrease soft tissue tension when excessive. Use of a constrained polyethylene liner is rarely needed in primary RSA. Finally, the short-stem and stemless RSA are very attractive, but longer-term data using such components is lacking.
Soft Tissue Tension
As mentioned previously, the ideal tension of the soft tissues for optimal function after RSA remains unknown. Most surgeons base their soft tissue tension on ease or difficulty of trial reduction and subjective intraoperative assessments of muscle tightness and passive range of motion. When RSA is implanted in a cuff-intact shoulder (i.e., primary osteoarthritis), the risk of dislocation secondary to lack of a posterior soft tissue restraint is less than in shoulders with posterosuperior cuff deficiency. As such, many surgeons favor a tighter RSA in FIRCT when compared to osteoarthritis. More objective ways to measure soft tissue tension intraoperatively are clearly needed.
Intraoperative Assessment of Perimeter Impingement
Once the final RSA configuration has been selected, it is important to eliminate potential remaining bony sources of impingement. On the glenoid side, this almost always involves removal of osteophytes identified as problematic during preoperative planning. On the humeral side, it is important to confirm lack of premature impingement between the greater tuberosity and the acromion-spine complex and between the lesser tuberosity and the coracoid or conjoined tendon. When perimeter impingement is identified in these locations despite optimal component selection and placement, bone may need to be trimmed from the greater and/or lesser tuberosity.
Subscapularis Management
This represents another major area of controversy. When the subscapularis has been chronically torn and is not repairable, the majority of surgeons do not try to mobilize the subscapularis or consider an anterior tendon transfer. However, when the subscapularis is intact at the time of surgical exposure, some surgeons repair the subscapularis whereas some leave the subscapularis unrepaired intentionally [26, 27]. To further complicate matters, rates of postoperative subscapularis healing after RSA are not completely understood [28]. Table 4 summarizes the potential advantages and disadvantages of subscapularis repair. Currently, the authors do try to repair the subscapularis whenever possible when RSA is performed for FIRCTs.
Table 4.
The subscapularis controversy
| Favors repair | Favors leaving unrepaired |
|---|---|
|
• Lower dislocation risk (deltopectoral approach, less lateralized implants) [26] • Potential for better internal rotation motion and strength |
• May antagonize deltoid and posterior cuff, limiting elevation and external rotation [29] • Prolongs operative time • Requires more postoperative protection • May contribute to coracoid impingement • Healing rates not well understood [28] |
The Role of Tendon Transfers as an Adjunct to RSA
Limited improvements in axial rotation were recognized early as a limitation of RSA. Additionally, deltoid dysfunction was initially considered as a contraindication for this procedure. Tendon transfers have been developed to improve motion and strength in external rotation and also to perform RSA in the setting of deltoid dysfunction.
Risk factors for poor postoperative active external rotation include extension of the posterior cuff tear to the teres minor and less lateralization. However, some authors have reported good restoration of active external rotation using a lateralized implant without adding tendon transfers [10]. Combined transfer of the latissimus dorsi and teres major or isolated transfer of the latissimus dorsi are the most common transfers considered (Fig. 3) [30, 31]. Transferring only the latissimus dorsi provides the potential of preserving more strength in internal rotation since the pectoralis major is not detached for harvest, and the teres major remains intact. The authors perform a transfer of the latissimus dorsi at the time of RSA for FIRCTs when there is active external rotation to less than neutral, no active external rotation with the arm in abduction (positive Hornsblower’s sign), and severe fatty infiltration of the teres minor. Adding the teres major to the transfer is considered for patients with a relatively weak or small latissimus dorsi (typically elderly females), especially if the subscapularis is preserved and can be repaired.
Fig. 3.
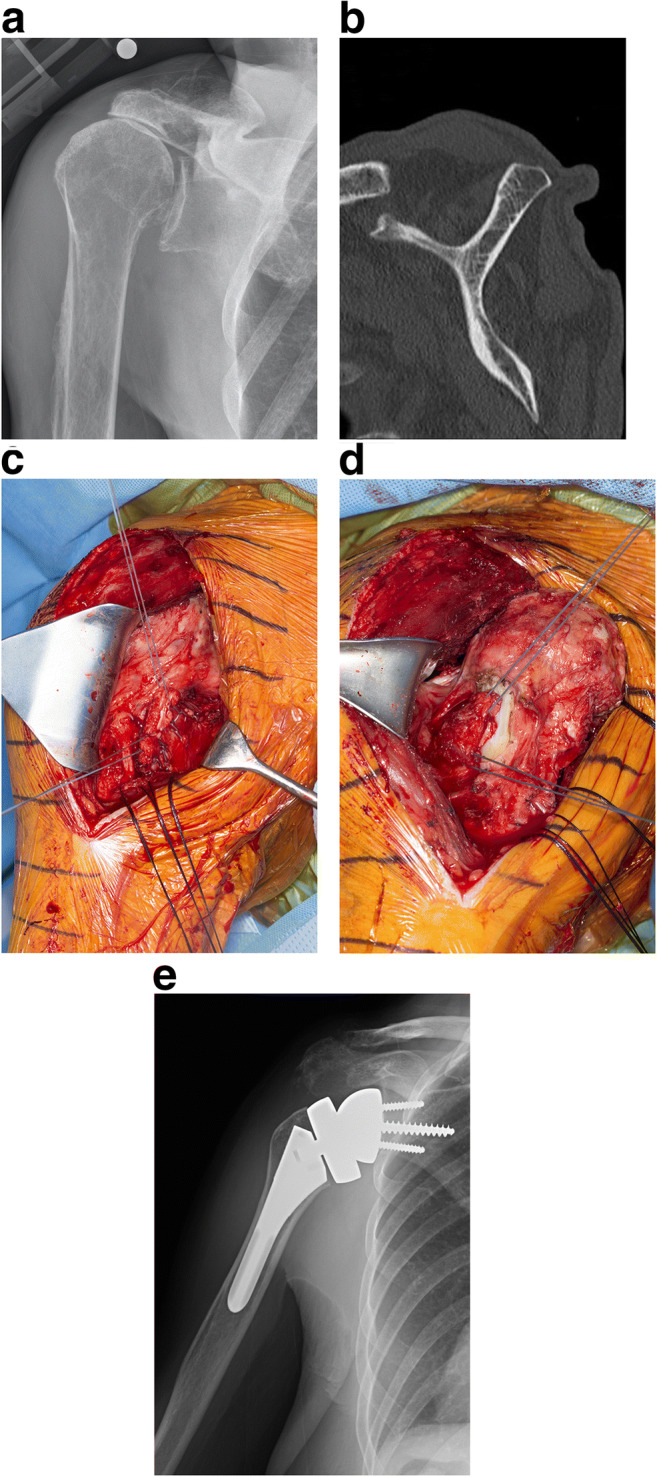
Latissimus dorsi transfer may be selectively required to optimize outcome when RSA is performed for a FIRCT. Preoperative radiograph (a) and CT scan (b) of a right shoulder with a FIRCT and severe atrophy and fatty infiltration of the teres minor. Harvest (c) and transfer (d) of the latissimus dorsi and teres major. e Postoperative radiograph
When the latissimus dorsi has been damaged in prior surgery (most often secondary to prior surgery), transfer of the lower trapezius may be considered at the time of RSA, although the results of this technique have only been reported as an isolated salvage procedure [7, 12], not in the setting of RSA. Additionally, when considering the lower trapezius transfer in RSA, a second incision is required to harvest the trapezius and an intercalary tendon graft is required to obtain enough length.
Regarding deltoid insufficiency, deficiency of the anterior third of the deltoid is not considered by many as a formal contraindication for RSA, but more extensive deltoid deficiency may increase the risk of dislocation and also limit gains in active elevation. Pedicled transfer of the pectoralis major muscle to compensate for deltoid insufficiency has been reported as relatively successful when combined with RSA in these circumstances [12].
Rehabilitation Programs
Immediately after surgery, a shoulder immobilizer is applied. There is little guidance regarding the ideal rehabilitation program after RSA [32]. When RSA is performed for FIRCT without subscapularis repair or tendon transfers, theoretically, early active range of motion should be safe. However, since the joint space is not sealed due to the absence of rotator cuff, immediate motion may increase the risk of hematoma. As such, our preference is to immobilize the shoulder for the first 2–4 weeks and then proceed with physical therapy. Even when the subscapularis is repaired, it could be argued that the semiconstrained nature of RSA may be more protective for subscapularis healing when motion is initiated early, but many surgeons recommend immobilization for 6 weeks when the subscapularis is repaired. Once range of motion and strengthening exercises are initiated, physical therapy exercises should be directed to strengthen the deltoid, periscapular muscles, and any remaining rotator cuff. When tendon transfers are performed in the setting of RSA, rehabilitation programs are different. Immobilization in external rotation for 6 weeks is recommended after latissimus dorsi transfer [31••]. After pedicled pectoralis transfer, the shoulder is immobilized in abduction for 6 to 8 weeks [12].
Summary
Multiple procedures have been described for the surgical management of FIRCT [7]. RSA is favored over other salvage procedures for older patients with lower expected functional demands, especially in the presence of anterosuperior escape, static anterior subluxation, articular joint pain, pan-circumferential tears, and cuff failure complicating anatomic TSA. Optimizing implantation of RSA in the cuff-deficient shoulder requires a balance between minimizing perimeter impingement and enhancing the function of the deltoid and any remaining rotator cuff. Tendon transfers at the time of RSA are beneficial in select patients, particularly those with absent external rotation secondary to extension of the posterosuperior cuff tear to the teres minor.
Funding
No funding was received.
Compliance with Ethical Standards
Conflict of Interest
Dr. Joaquin Sanchez-Sotelo, MD PhD, receives royalties from Stryker, consulting fees from Exactech and Wright Medical, publication royalties from Elsevier and Oxford University Press, and an honorarium from Journal of Shoulder and Elbow Surgery.
Dr. George S. Athwall MD receives royalties from Conmed and consulting fees from Exactech and Wright Medical.
Footnotes
This article is part of the Topical Collection on Surgical Management of Massive Irreparable Cuff Tears
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
- 1.Grammont PM, Baulot E. Delta shoulder prosthesis for rotator cuff rupture. Orthopedics. 1993;16(1):65–68. doi: 10.3928/0147-7447-19930101-11. [DOI] [PubMed] [Google Scholar]
- 2.Kennon, J.C., Songy C., Bartels D., Statz J., Cofield R.H., Sperling J.W., Sanchez-Sotelo J., Primary reverse shoulder arthroplasty: how did medialized and glenoid-based lateralized style prostheses compare at 10 years? J Shoulder Elb Surg, 2020. [DOI] [PubMed]
- 3.Mulieri P, Dunning P, Klein S, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of irreparable rotator cuff tear without glenohumeral arthritis. J Bone Joint Surg Am. 2010;92(15):2544–2556. doi: 10.2106/JBJS.I.00912. [DOI] [PubMed] [Google Scholar]
- 4.Hartzler RU, et al. Reverse shoulder arthroplasty for massive rotator cuff tear: risk factors for poor functional improvement. J Shoulder Elbow Surg. 2015;24(11):1698–1706. doi: 10.1016/j.jse.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Barco R, Savvidou OD, Sperling JW, Sanchez-Sotelo J, Cofield RH. Complications in reverse shoulder arthroplasty. EFORT Open Rev. 2016;1(3):72–80. doi: 10.1302/2058-5241.1.160003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Werthel JD, et al. Lateralization in reverse shoulder arthroplasty: a descriptive analysis of different implants in current practice. Int Orthop. 2019;43(10):2349–2360. doi: 10.1007/s00264-019-04365-3. [DOI] [PubMed] [Google Scholar]
- 7.Burnier M, Elhassan BT, Sanchez-Sotelo J. Surgical management of irreparable rotator cuff tears: what works, what does not, and what is coming. J Bone Joint Surg Am. 2019;101(17):1603–1612. doi: 10.2106/JBJS.18.01392. [DOI] [PubMed] [Google Scholar]
- 8.Gilbart MK, Gerber C. Comparison of the subjective shoulder value and the constant score. J Shoulder Elb Surg. 2007;16(6):717–721. doi: 10.1016/j.jse.2007.02.123. [DOI] [PubMed] [Google Scholar]
- 9.Boileau P, Baba M, McClelland WB, Jr, Thélu CÉ, Trojani C, Bronsard N. Isolated loss of active external rotation: a distinct entity and results of L'Episcopo tendon transfer. J Shoulder Elb Surg. 2018;27(3):499–509. doi: 10.1016/j.jse.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Berglund DD, Rosas S, Triplet JJ, Kurowicki J, Horn B, Levy JC. Restoration of external rotation following reverse shoulder arthroplasty without latissimus dorsi transfer. JB JS Open Access. 2018;3(2):e0054. doi: 10.2106/JBJS.OA.17.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elhassan BT, Alentorn-Geli E, Assenmacher AT, Wagner ER. Arthroscopic-assisted lower trapezius tendon transfer for massive irreparable posterior-superior rotator cuff tears: surgical technique. Arthrosc Tech. 2016;5(5):e981–e988. doi: 10.1016/j.eats.2016.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elhassan BT, Wagner ER, Werthel JD, Lehanneur M, Lee J. Outcome of reverse shoulder arthroplasty with pedicled pectoralis transfer in patients with deltoid paralysis. J Shoulder Elb Surg. 2018;27(1):96–103. doi: 10.1016/j.jse.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Salazar DH, Chalmers PN, Mackinnon SE, Keener JD. Reverse shoulder arthroplasty after radial-to-axillary nerve transfer for axillary nerve palsy with concomitant irreparable rotator cuff tear. J Shoulder Elb Surg. 2017;26(1):e23–e28. doi: 10.1016/j.jse.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 14.Friedman RJ, Barcel DA, Eichinger JK. Scapular notching in reverse total shoulder arthroplasty. J Am Acad Orthop Surg. 2019;27(6):200–209. doi: 10.5435/JAAOS-D-17-00026. [DOI] [PubMed] [Google Scholar]
- 15.Lévigne C, Boileau P, Favard L, Garaud P, Molé D, Sirveaux F, Walch G. Scapular notching in reverse shoulder arthroplasty. J Shoulder Elb Surg. 2008;17(6):925–935. doi: 10.1016/j.jse.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 16.Duethman NC, Aibinder WR, Nguyen NTV, Sanchez-Sotelo J. The influence of glenoid component position on scapular notching: a detailed radiographic analysis at midterm follow-up. JSES Int. 2020;4(1):144–150. doi: 10.1016/j.jses.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lieber RL, Roberts TJ, Blemker SS, Lee SSM, Herzog W. Skeletal muscle mechanics, energetics and plasticity. J Neuroeng Rehabil. 2017;14(1):108. doi: 10.1186/s12984-017-0318-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamilton MA, Diep P, Roche C, Flurin PH, Wright TW, Zuckerman JD, Routman H. Effect of reverse shoulder design philosophy on muscle moment arms. J Orthop Res. 2015;33(4):605–613. doi: 10.1002/jor.22803. [DOI] [PubMed] [Google Scholar]
- 19.Lädermann A, Chiu JCH, Cunningham G, Hervé A, Piotton S, Bothorel H, Collin P. Do short stems influence the cervico-diaphyseal angle and the medullary filling after reverse shoulder arthroplasties? Orthop Traumatol Surg Res. 2020;106(2):241–246. doi: 10.1016/j.otsr.2019.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Iannotti JP, Walker K, Rodriguez E, Patterson TE, Jun BJ, Ricchetti ET. Accuracy of 3-dimensional planning, implant templating, and patient-specific instrumentation in anatomic total shoulder arthroplasty. J Bone Joint Surg Am. 2019;101(5):446–457. doi: 10.2106/JBJS.17.01614. [DOI] [PubMed] [Google Scholar]
- 21.Boileau P, Moineau G, Roussanne Y, O’Shea K. Bony increased offset-reversed shoulder arthroplasty (BIO-RSA) JBJS Essent Surg Tech. 2017;7(4):e37. doi: 10.2106/JBJS.ST.17.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iannotti JP, McCarron J, Raymond CJ, Ricchetti ET, Abboud JA, Brems JJ, Williams GR. Agreement study of radiographic classification of rotator cuff tear arthropathy. J Shoulder Elb Surg. 2010;19(8):1243–1249. doi: 10.1016/j.jse.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Friedman RJ, Hawthorne KB, Genez BM. The use of computerized tomography in the measurement of glenoid version. J Bone Joint Surg Am. 1992;74(7):1032–7. doi: 10.2106/00004623-199274070-00009. [DOI] [PubMed] [Google Scholar]
- 24.Boileau P, Gauci MO, Wagner ER, Clowez G, Chaoui J, Chelli M, Walch G. The reverse shoulder arthroplasty angle: a new measurement of glenoid inclination for reverse shoulder arthroplasty. J Shoulder Elb Surg. 2019;28(7):1281–1290. doi: 10.1016/j.jse.2018.11.074. [DOI] [PubMed] [Google Scholar]
- 25.Gutiérrez S, et al. Range of impingement-free abduction and adduction deficit after reverse shoulder arthroplasty. Hierarchy of surgical and implant-design-related factors. J Bone Joint Surg Am. 2008;90(12):2606–2615. doi: 10.2106/JBJS.H.00012. [DOI] [PubMed] [Google Scholar]
- 26.Matthewson G, Kooner S, Kwapisz A, Leiter J, Old J, MacDonald P. The effect of subscapularis repair on dislocation rates in reverse shoulder arthroplasty: a meta-analysis and systematic review. J Shoulder Elb Surg. 2019;28(5):989–997. doi: 10.1016/j.jse.2018.11.069. [DOI] [PubMed] [Google Scholar]
- 27.Rol M, Favard L, Berhouet J. Factors associated with internal rotation outcomes after reverse shoulder arthroplasty. Orthop Traumatol Surg Res. 2019;105(8):1515–1519. doi: 10.1016/j.otsr.2019.07.024. [DOI] [PubMed] [Google Scholar]
- 28.Dedy NJ, Gouk CJ, Taylor FJ, Thomas M, Tan SLE. Sonographic assessment of the subscapularis after reverse shoulder arthroplasty: impact of tendon integrity on shoulder function. J Shoulder Elb Surg. 2018;27(6):1051–1056. doi: 10.1016/j.jse.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Giles JW, Langohr GDG, Johnson JA, Athwal GS. The rotator cuff muscles are antagonists after reverse total shoulder arthroplasty. J Shoulder Elb Surg. 2016;25(10):1592–1600. doi: 10.1016/j.jse.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 30.Puskas GJ, Catanzaro S, Gerber C. Clinical outcome of reverse total shoulder arthroplasty combined with latissimus dorsi transfer for the treatment of chronic combined pseudoparesis of elevation and external rotation of the shoulder. J Shoulder Elb Surg. 2014;23(1):49–57. doi: 10.1016/j.jse.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 31.Boileau P, et al. Modified latissimus dorsi and teres major transfer through a single delto-pectoral approach for external rotation deficit of the shoulder: as an isolated procedure or with a reverse arthroplasty. J Shoulder Elbow Surg. 2007;16(6):671–682. doi: 10.1016/j.jse.2007.02.127. [DOI] [PubMed] [Google Scholar]
- 32.Kirsch JM, Namdari S. Rehabilitation after anatomic and reverse total shoulder arthroplasty: a critical analysis review. JBJS Rev. 2020;8(2):e0129. doi: 10.2106/JBJS.RVW.19.00129. [DOI] [PubMed] [Google Scholar]