Abstract
Objective
Although prolonged mechanical ventilation (PMV) is increasingly common, little is known concerning patient symptom burden or attitudes toward PMV. This study aims to describe the mood, well-being, distressing symptoms, and attitudes toward prolonged ventilation among PMV patients treated either at home or long-term acute care (LTAC).
Design
An observational study.
Setting and Participants
62 communicative participants treated with PMV, aged ≥18 years, insurees of a single HMO, treated at home hospital or LTAC specializing in ventilation in Jerusalem.
Measures
Sociodemographic characteristics; chronic conditions; functional status; symptom burden measured by revised Edmonton Symptomatic Assessment System (r-ESAS); attitudes toward PVM.
Results
Participants were aged 61.7 ± 20.7 years, commonly suffered progressive neuromuscular disease (43.5%) or chronic lung disease (29%), were functionally dependent, treated at home (64.5%) or LTAC (35.5%), and had a mean PMV duration of 36.6 months (interquartile range 10.8-114.1). The 5-item, short Geriatric Depression Scale identified depression among 38% of participants, and was less at home vs LTAC (34% vs 44%, P < .001). Mean revised Edmonton Symptom Assessment System score was 24.5 ± 14.8 (maximum severity = 100), and participants reported severe or distressing symptoms for tiredness (27%/20%), pain (10%/25%), anxiety (16%/14%), depression (9%/21%), drowsiness (12%/17%), shortness of breath (9%/15%), poor appetite (7%/9%), and nausea (0%/10%). Impaired general well-being was reported as severe, moderate, mild, or none among 15%, 40%, 30%, and 15%, respectively. Only 1 patient had advance directives concerning ventilation prior to intubation, and when asked if they had to choose again today, 85% of patients would again opt for ventilation.
Conclusions and Implications
Few PMV patients reported distressing symptoms, and 85% would choose ventilation if asked again. These findings might be useful in clinical practice to assist in decision making concerning prolonged ventilation.
Keywords: Prolonged mechanical ventilation, patient-reported outcomes, symptom assessment, patient attitudes to ventilation, decisional regret
There is a steady increase in the number of people treated with prolonged mechanical ventilation (PMV), commonly defined as >6 hours daily for >3 weeks, with an estimated 400,000 PMV patients treated in the United States during 2019.1, 2, 3, 4, 5 Advances in technological sophistication of respiratory supportive care, alongside the increasing availability of relatively simple-to-use ventilator systems, are responsible for the widening clinical spectrum of patients treated with PMV. Similarly, as the availability of sophisticated models for home care increases, a growing number of PMV patients choose to remain at home, which is often a preferred alternative to prolonged care in a specialized long-term care facility.6, 7, 8, 9, 10, 11, 12 Patients requiring PMV include those who fail to wean from ventilator following an acute event such as acute respiratory failure, acute brain injury, and cardiopulmonary resuscitation, alongside survivors of intensive care units suffering from chronic critical care illness.13 , 14 In addition, there is a growing population of PMV patients with progressive neuromuscular diseases and chronic lung diseases who opt for semielective ventilation when faced with encroaching respiratory failure.
Research into PMV has primarily focused on objective measures and outcomes of care including long-term survival, rates of long-term liberation from ventilation, and economic ramifications of care.2 , 4 , 15, 16, 17 Despite the importance of the increasing population of PMV patients as a public health care challenge, far less is known about the patient's perspective toward PMV. Although limited research does exist concerning overall quality of life,18, 19, 20, 21, 22 little is known about the range and severity of symptomatology, and only rarely has research addressed the attitudes of PMV patients themselves toward prolonged ventilation, and their decisional regret. In light of the recent increase in the number of people receiving invasive ventilation due to COVID-19, often for a prolonged period of time, this area is of particular relevance.
This observational study among PMV patients aims to describe the range and severity of common symptoms, their mood and well-being, as well as their attitudes concerning ventilation.
Methods
Study Design
The current study focuses on a subset of 62 participants treated with PMV and able to communicate, who were enrolled in a larger observational study of 120 invasive PMV patients (defined as ventilation via tracheostomy ≥21 days), treated either at home or in long-term acute care (LTAC) between May 1, 2016, and April 31, 2018. A comprehensive description of the study methodology and patient characteristics has been recently published.23
Setting
Study participants were all insurees of the Clalit Health Services, the largest HMO in Israel, which provides mandatory comprehensive health coverage to all citizens. PMV care in Jerusalem is provided either in a single specialized LTAC (Chronic Ventilator-Dependent Division, Herzog Medical Center, Jerusalem), or at home, cared for by the Jerusalem Home Hospital.24, 25, 26, 27, 28 The decision concerning site of care depends on the patient's and/or custodian's agreement, sufficient informal/formal home care, and medical condition. At the time of the study, there were 120 beds in the LTAC available for adult PMV patients, of which 76 beds were occupied by PMV patients of the Clalit Health Fund. During the study period, there were 47 adult PMV patients cared for at home by the Jerusalem Home Hospital. Both settings provide comprehensive multidisciplinary team, with 24/7 on-call medical and respiratory backup at home, and on-site medical, nursing, and respiratory care in LTAC.23
Participants
All adult invasive PMV participants (aged ≥18 years, Clalit Health Services insurees) treated either at home or in LTAC during the study period in Jerusalem were eligible for inclusion in the study and all were approached to enroll. Those participants already being treated with PMV at the start of the study were approached to enroll, as were subsequent participants who were admitted to LTAC or home hospital during the study period. Informed consent was given by cognitively intact participants or legal custodians where appropriate. We identified a total of 123 potential participants (47 at home and 76 in LTAC), among whom 1 communicative patient (at home) and 2 legal custodians of noncommunicative patients (at LTAC) declined to enroll. Among the 120 enrolled participants, there were a total of 62 participants who were able to communicate (40 at home, 22 at LTAC), all of whom gave their consent to enroll. The current study focuses exclusively on this subset of 62 communicative PMV participants. The local ethics committee approved the study proposal.
Data Collection
Data were collected from review of medical records and structured patient interview by the study assistant. Medical records were available for all participants, and all participants were interviewed. Data were confidentially coded with a unique identifier and uploaded to the secure research database.
Measures
Standardized questionnaire included sociodemographic characteristics, primary indication for PMV, current comorbidities and medications, functional status before and after PMV according to the Barthel index29 , 30 (measuring dependence in eating, bathing, personal hygiene, and dressing, continence of bladder and bowel, toileting, transfer from bed to chair; mobility, and stair climbing, with total independence maximum score = 100), daily hours, and duration of ventilation.
Symptom Assessment
Assessment of patient symptoms was performed using the revised Edmonton Symptom Assessment System (r-ESAS) (a 10-item set of patient-reported outcomes assessing the current feeling of tiredness, lack of appetite, pain, drowsiness, nausea, shortness of breath, overall well-being, depression, anxiety, and other problems). Each symptom is graded by patient from 0 (no problem) to 10 (most severe), with the total score ranging from 0 to 100 (100 most severe).31 , 32 As regards the definition of well-being, the participants were instructed to rate their overall feeling of health in general, from 0 (best) to 10 as the worst possible feeling. The r-ESAS has been validated in 20 languages including Hebrew.33 The 5-item, short Geriatric Depression Scale (GDS)34 was used to determine depression, with each question scored as 0 or 1 for a maximum score of 5, with depression defined as ≥2/5.
Attitudes Toward Ventilation
Participants answered the following question: “If you had to decide today concerning advance directives for long term ventilation, how would you choose?”, the possible answers being “yes, I would choose to be ventilated,” “no, I would choose not to be ventilated,” or “unsure.”
Statistical Methods
For continuous variables, we determined means and standard deviations or median and interquartile range, as appropriate. The total r-ESAS score and attitude toward ventilation were analyzed by site of care and age of patient (cutoff chosen by age median). Comparisons were performed using chi-square, t test, or Wilcoxon rank-sum test.
Multivariate analysis, using a linear regression, examined the relationship between r-ESAS score and independent variables, including age, gender, marital status, financial difficulty, site of care, cause of PMV (chronic lung disease, degenerative neuromuscular disease, acute cause), and functional status. Multivariate analysis using logistic regression examined the relationship between depression (measured by the 5-item GDS) and these variables. Demographic, medical, and functional variables were tested. The multivariate model included variables with P < .2 in univariate testing.
Results
Patient Characteristics
Baseline characteristics are shown in Table 1 for the 62 PMV study participants. Mean age was 61.7 ± 20.8 years, 34% were single, 21% had >12 years' education, 52% had no legal custodian, and participants were more frequently cared for at home (64.5%) vs in LTAC (35.5%). The most common indication for PMV was progressive degenerative neuromuscular disease (43.5%) followed by chronic lung disease (29%). Prior to PMV, the mean Barthel index score was 60.4 ± 34.2, with a mean decline in score of 41 ± 30.3 following PMV. Most participants were ventilated >18 hours daily, and the mean duration of PMV was 36.6 months [interquartile range (IQR) 10.8-114.1] at the study end.
Table 1.
Baseline Characteristics of PMV Patients (N = 62)
| Characteristic | Communicative Patients |
|---|---|
| Age, mean (SD) | 61.7 (20.7) |
| Gender | |
| Male | 34 (54.8) |
| Female | 28 (45.2) |
| Marital status | |
| Married | 30 (48.4) |
| Single | 21 (33.9) |
| Divorced | 2 (3.2) |
| Widow | 9 (14.5) |
| Number of children, median (IQR) | 1.5 (0-5) |
| Financial difficulty (self-reported) | 22 (36.7) |
| Education | |
| 0–12 y | 40 (65.6) |
| >12 y | 21 (34.4) |
| Legal custodian: none | 32 (51.6) |
| Site of care | |
| Home | 40 (64.5) |
| Long-term care | 22 (35.5) |
| Cause for ventilation | |
| Neurologic acute∗ | 10 (16.1) |
| Neurologic degenerative† | 27 (43.5) |
| Chronic lung disease | 18 (29) |
| Heart disease | 3 (4.8) |
| Post sepsis | 9 (14.5) |
| Other | 8 (12.9) |
| Number of chronic diseases, mean (SD)‡ | 1.68 (1.80) |
| Functional status | |
| Barthel Index prior to PMV, mean (SD) | 60.4 (34.2) |
| Barthel Index change post PMV, §mean (SD) | 41 (30.3) |
| Medications | |
| Antidepressants | 21 (33.9) |
| Antipsychotics | 15 (24.2) |
| Pain | 17 (27.4) |
| Sedatives | 21 (33.9) |
| Daily ventilation | |
| <12 h/d | 5 (8.1) |
| 12–18 h/d | 11 (17.7) |
| >18 h/d | 46 (74.2) |
| Duration of PMV at study end, mo, median (IQR) | 36.6 (10.8-114.1) |
Unless otherwise noted, values are n (%).
Acute neurologic causes, for example, post cerebrovascular accident, post head trauma, status post resuscitation.
Degenerative neurologic causes, for example, amyotrophic lateral sclerosis, progressive neuromuscular diseases.
Number of chronic diseases from the following: diabetes mellitus, ischemic heart disease, chronic heart failure, hypertension, chronic lung disease, chronic renal failure, dementia, past history of cancer, past history of stroke.
Barthel index score: 0 = maximum independence, 100 = maximum dependence.
Symptom Assessment
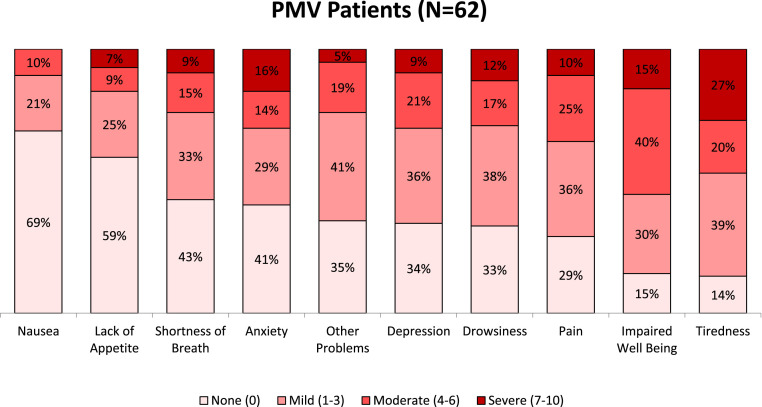
The scores for each of the 10 items of the r-ESAS are shown in Table 2 . The total mean r-ESAS score was 24.5 ± 14.8 (maximum score 100), and both the mean and median score for all individual subitems was <4 (maximum score 10), apart from the subitem for general well-being (mean 3.8 ± 2.6, median 4, IQR 2-6). The distribution of severity of symptoms is shown in Figure 1 , using cutoff points to define each subitem as asymptomatic (score 0), mild (score 1-3), moderate (score 4-6), and severe (score 7-10). Participants reported severe or distressing symptoms for tiredness (27%/20%), pain (10%/25%), anxiety (16%/14%), depression (9%/21%), drowsiness (12%/17%), shortness of breath (9%/15%), poor appetite (7%/9%), and nausea (0%/10%). Accordingly, many participants were asymptomatic or mildly symptomatic for nausea (90%), poor appetite (84%), shortness of breath (76%), drowsiness (71%), depression (70%), anxiety (70%), pain (65%), and tiredness (53%). Impaired general well-being was reported as severe, moderate, mild, or none among 15%, 40%, 30%, and 15%, respectively. In addition to the single item for depression in the r-ESAS, the short-form 5-item GDS revealed a mean score of 1.32 ± 1.36, with 38% of participants screening positive for depressive symptomatology, scoring ≥2/5. Interestingly 33% of participants were receiving antidepressant medication, whereas the use of analgesics and sedatives was 27% and 34%, respectively. Despite a trend toward less symptom burden among younger participants, there were no significant differences in the total r-ESAS score among participants aged <65 years vs those aged ≥65 years, with a mean score of 21.2 (SD 12.5) vs 27.9 (SD 16.2) (P = .08), and a median score of 19 (IQR 14-31) vs 28 (IQR 20-33) (P = .10). Several multivariate analyses were performed to examine the possible association between baseline independent variables and either depression or the total sum r-ESAS score. No significant association was observed with depression in several logistic regression analyses; however, a significant association between chronic lung disease and increased r-ESAS score was observed in linear regression analyses (beta coefficient 14, P < .01).
Table 2.
Common Symptoms Among PMV Patients
| Symptoms | Mean (SD) | Median (IQR); (Minimum, Maximum) |
|---|---|---|
| r-ESAS total score (0-100) | 24.5 (14.8) | 22.5 (14-33); (0, 84) |
| Subitem score (0-10) | ||
| 1. Tiredness | 4 (2.9) | 3 (2-7); (0, 10) |
| 2. Lack of appetite | 1.6 (2.4) | 0 (0-3); (0, 10) |
| 3. Pain | 2.7 (2.5) | 3 (0-5); (0, 9) |
| 4. Drowsiness | 2.4 (2.7) | 1.5 (0-4); (0, 9) |
| 5. Depression | 2.3 (2.5) | 2 (0-4); (0, 9) |
| 6. Nausea | 0.9 (1.7) | 0 (0-2); (0, 6) |
| 7. Shortness of breath | 2.1 (2.7) | 1 (0-3); (0, 10) |
| 8. Impaired well-being | 3.8 (2.6) | 4 (2-6); (0, 10) |
| 9. Anxiety | 2.4 (2.8) | 1 (0-4); (0, 9) |
| 10. Other problems | 2.1 (2.4) | 1.5 (0-3); (0, 10) |
| Depression∗ | ||
| Short GDS score | 1.32 (1.36) | 1 (0-2); (0, 5) |
| Frequency of depression,† n (%) | 19 (38) | |
| Frequency of each GDS question, n (%) | ||
| Not satisfied with your life | 10 (20) | |
| Often get bored | 20 (40) | |
| Feel helpless | 16 (32) | |
| Prefer to stay at home | 12 (24) | |
| Feel pretty worthless | 8 (16) | |
The r-ESAS consists of 10 items, each graded 0-10, with 0 = no symptoms and 10 = maximum distress. The total score thus ranges from 0 (completely symptom free) to 100 (most severe).
n = 50 for the 5-item GDS scoring.
Depression defined as a score ≥2/5 using the 5-item short GDS.
Fig. 1.
Severity of symptoms among PMV patients, assessed using the revised Edmonton Symptom Assessment Scale. Each item of the r-ESAS is graded 0-10, with 0 = no symptoms and 10 = maximum distress.
Site of Care and Symptom Assessment
We re-examined the data according to site of care, comparing participants treated at home (40/62) to those in LTAC (22/62). The symptom burden of PMV patients was similar irrespective of site of care, with a mean r-ESAS score of 24.9 ± 16.7 vs 23.7 ± 10.0 (P = .74) at home and LTAC, respectively. Although no significant differences were observed in the severity of the 10 r-ESAS subitem symptoms between home and LTAC, nonetheless, well-being was reported as asymptomatic or mildly impaired by 54% of home PMV patients compared with 26% among long-term care, with similar rates of severely impaired well-being reported by 10% and 11% of patients, respectively. Depressive symptomatology, as measured using the 5-item short GDS, was less frequent at home compared with LTAC (34% vs 44%, P = .049). Planned ventilation was more common among participants at home hospital, and the initiation of ventilation (prior to intubation) was discussed with the patient or family members and/or custodians among 74% vs 43% (P = .001) of home vs LTAC PMV patients, respectively. No other significant differences were observed.
Advance Directives and Preferences Concerning Ventilation
As shown in Table 3 , only 85.5% (53/62) of participants were initially intubated within a hospital setting, and among 30.6% (19/62) the intubation was elective. Prior discussion with either patient, family, or custodian concerning intubation was reported to have taken place among 67.2% of study participants. Only 1 of the 62 study participants had formal advance directives concerning ventilation prior to the initiation of ventilation. In answer to the question “If you had to decide today concerning advance directives for ventilation, how would you choose?” 7 of 61 participants (11.5%) answered that they would choose not to be ventilated, compared to 52/61 (85.2%) who would choose again to be ventilated, and 2 participants who were undecided. Among the 19 elective PMV participants, discussion concerning ventilation prior to intubation was reported by 15 participants, no discussion reported by 1, and 3 failed to answer the question. Among these 15 participants who discussed PMV prior to elective intubation, 13/15 (86.7%) answered that they would choose again today in favor of ventilation.
Table 3.
Advance Directives and Attitudes Toward Ventilation (N = 62)
| Study Participants, n (%) | |
|---|---|
| Site of initial intubation | |
| Outside hospital | 9 (14.5) |
| Hospital or long-term care | 53 (85.5) |
| Urgency of ventilation | |
| Elective | 19 (30.6) |
| Urgent | 43 (69.4) |
| Was ventilation discussed prior to intubation? | |
| No | 19 (32.8) |
| Yes | 39 (67.2) |
| Were there advanced directives concerning ventilation prior to intubation? | |
| No | 59 (98.3) |
| Yes | 1 (1.7) |
| “If you had to decide today concerning advanced directives for long term ventilation, how would you choose?” | |
| No | 7 (11.5) |
| Yes | 52 (85.2) |
| Undecided | 2 (3.3) |
There were no significant differences observed concerning choices around ventilation when subdividing the study sample according to age <65 years vs those aged ≥65 years old, with 85.7% (24/29) vs 84.8% (28/33) in favor of PMV (P = .8), respectively. Similarly, no significant differences were observed when subdividing participants according to home vs LTAC, with 82.2% (32/40) vs 90.9% (20/22) in favor of PMV (P = .9), respectively.
Discussion
This study describes the mood, symptoms, well-being, and attitudes toward ventilation, among study participants treated with prolonged mechanical ventilation via tracheostomy both at home and in LTAC. Progressive neuromuscular diseases, chronic lung disease and chronic critical illness where the most common causes for PMV, and participants were typically highly functionally dependent, ventilated >18 hours daily, with a mean duration of PMV of around 3 years. Few participants reported moderate or severe distress across a wide range of symptoms, both physical (nausea, poor appetite, shortness of breath, drowsiness, tiredness, and pain) and emotional (depression and anxiety), whereas the global measure of general well-being was reported as either normal or mild impairment among 45%, moderately impaired among 40%, and severely impaired among 15% of participants. Half of those cared for at home reported their overall well-being as normal or only mildly impaired, compared with only a quarter of LTAC, and, similarly, depression was significantly less common at home vs LTAC (34% vs 44%). Whereas advance directives were completely lacking prior to initiation of ventilation, an interesting finding to emerge was the observation that if faced again with the decision to choose PMV, then 85% of patients would opt for PMV. Research into the health-related quality of life among patients receiving PMV has commonly focused on patients treated with noninvasive ventilation, with assessment based on either single-item questions or tools such as the EQ-5D (EuroQol-5 Dimensions)35 or more recently the Severe Respiratory Insufficiency questionnaire.36 This 49-item test has typically been used among patients with advanced lung disease, as well as those treated with noninvasive ventilation,37 and evaluates the impact of respiratory insufficiency on several aspects of health-related quality of life including respiratory complaints, physical function, attendant symptoms, social relationships, anxiety, psychological well-being, and social functioning. Findings have varied considerably depending on the patient population, with more severe symptoms described among older patients with chronic lung disease as compared to younger patient population with progressive neuromuscular disease. The r-ESAS scale used in the present study has been widely used in both clinical practice and research for more than 25 years and repeatedly shown to be a robust and validated tool in the measurement of patient-reported outcome measures.33 Originally designed to assess the palliative needs of oncology patients, it has seen widespread use among numerous other patient groups, including, for example, nephrology, cardiac, hepatic diseases, multiple chronic comorbidities, and pulmonary diseases,33 , 38 in both inpatient and outpatient ambulatory and home care settings. Extensive research and comparative studies have validated meaningful cutoff points to define asymptomatic, mild, moderate, or severe symptomatology, and which we used in this study.32 , 39 , 40 Accordingly, a score greater than ≥7/10 on any individual subitem has been defined by some researchers as a high symptom burden, and suggested to serve as a “trigger” in clinical practice to highlight the need for reassessment of palliative care needs.
Our data help quantify the severe distress experienced by PMV patients in the study sample, among whom severe (≥7/10) anxiety, drowsiness, tiredness, and impaired well-being were reported by 16%, 12%, 27%, and 15%, with the remaining symptoms reported as severe by ≤ 10% of patients. Similarly, frequency of depression was 38%, which is considerably higher than the estimates of 20% baseline rate among the local Jerusalem population aged 70 years41 and 25% among a national sample aged ≥65 years.42 Indeed, the depression rates of our sample are similar to residents of long-term care facilities.43
A prerequisite first step toward relieving this distress is the need to increase awareness among health care workers and provide skills to proactively identify distressing symptoms shown here to affect PMV patients.
Although few patients suffered severe levels of distressing symptoms, the range of symptom severity observed among our sample was very similar to a study of 506 community-based patients (nonventilated) enrolled into a home care unit.40 Among patients in our study, the severity of most symptoms was unaffected by the site of care (whether at home or in long-term care). A similar finding was also reported in a study of 32 invasive PMV patients treated either at home or nursing facility, among whom the reported health-related quality of life ranged from very good (primarily among the 14 neuromuscular disease patients) to poor (among older chronic lung disease) irrespective of place.18 Interestingly, the only significant association to emerge from several multivariate analysis of our data was the association between chronic lung disease and an increase in the total r-ESAS score, mediated by the r-ESAS subitem of increased severity of the shortness of breath. Among the few studies that have addressed this point, this would appear to be a recurrent finding for both invasive and noninvasive PMV patients.18 , 19
Qualitative research into the impact of invasive PMV among young men with Duchenne muscular dystrophy in Denmark,20 and patients aged between 18 and 75 years with various neuromuscular diseases from Norway21 emphasized the recurrent themes of “empowerment” and the “positive contribution to a purposeful meaningful life” expressed by patients. Of note, in answer to one of the items in the short GDS, 80% of the patients in our study answered that “overall they were satisfied with their life,” which is in accordance with the assessment of overall well-being among the patients in our study. The positive perspective on overall health and well-being has also been noted in several studies of home PMV patients,22 and although it may reflect high quality of care, it highlights the dissonance between good subjective patient-reported outcomes, on the one hand, and their extremely complex medical needs, functional impairment, and high technological dependence, on the other.
The decision-making process surrounding PMV is complex. It is noteworthy that many study participants suffered from progressive diseases for which respiratory failure was a foreseeable event, often a long time in advance. Nonetheless, all but 1 study participant were without advance directives at the time of intubation. The observation that 85% of our study participants would, if necessary, again choose ventilation, is in keeping with the few studies that have also addressed this sensitive issue. Despite different patient populations, different place of care, and different treatment models, the percentages of patients stating they would again choose ventilation were similar: 84.7% among 315 PMV patients in LTAC in the United States (of whom 54% were successfully weaned at 1 year, and the total 1-year survival rate was 66.9%);44 90% among 77 patients with both lung and neuromuscular diseases treated with home PMV via tracheostomy in Italy, median survival 49 months;45 85% of 19 patients with neuromuscular diseases treated at home in the United States, median survival 54 months;46 8/14 (57.1%) of COPD patients and 9/11 (81.8%) of progressive neuromuscular disease patients with invasive PMV at home in the United States.19 The consistency of this finding attests to its validity and affirms that the findings of our study are reproducible and thus representative of other similar patient populations. Similarly, comparisons of data describing similar patient populations from other countries, treated either in long-term care or at home, suggest that our findings can be extrapolated to other populations of PMV patients.9 , 11 , 12 , 16 , 44
Recent work5 that aimed to introduce algorithms to aid the informed decision-making process concerning PMV have largely failed to change the pattern of decisions, and a frequent finding to emerge has been the “overoptimistic” outlook of the health proxy.
Limitations
A possible source of bias may have been introduced, because all patients were treated by a single center, and therefore local standards and practices may have influenced the findings. Our research is limited by its inclusion of only patients who were able to communicate. The patients who declined ventilation, and those who did not survive long enough to reach prolonged ventilation, as well PMV patients unable to communicate—the opinion and voice of these “nonsurvivors” and silent patients remain unheard. However, as suggested elsewhere, the survivors of prolonged ventilation are the closest witnesses of the “struggle endured,” and as such may speak by proxy in the light of their own experience.47 , 48
Conclusions and Implications
Few patients with PMV reported distressing symptoms, depression, or impaired well-being. Most patients did not regret their decision to undergo PMV. These findings deserve consideration and may assist decision making concerning prolonged ventilation.
Acknowledgments
We acknowledge Ruth Steiner (RN) for the many hours spent collecting data and interviewing the patients and their caregivers both in the LTAC and at home. We also acknowledge Aliza Hammerman-Rozenberg (MA) for her statistical consultation.
Footnotes
J.M.J. and E.-L.M. contributed equally as first authors.
This research was funded by a grant (number 185/2014) from the National Institute for Health Policy Research, Israel. The funding body had no role in the design or conduct of the research, interpretation of the data, or preparation of the manuscript.
The authors declare no conflicts of interest.
References
- 1.MacIntyre N.R., Epstein S.K., Carson S. Management of patients requiring prolonged mechanical ventilation: Report of a NAMDRC consensus conference. Chest. 2005;128:3937–3954. doi: 10.1378/chest.128.6.3937. [DOI] [PubMed] [Google Scholar]
- 2.Zilberberg M.D., Luippold R.S., Sulsky S. Prolonged acute mechanical ventilation, hospital resource utilization, and mortality in the United States. Crit Care Med. 2008;36:724–730. doi: 10.1097/CCM.0B013E31816536F7. [DOI] [PubMed] [Google Scholar]
- 3.Zilberberg M.D., de Wit M., Shorr A.F. Accuracy of previous estimates for adult prolonged acute mechanical ventilation volume in 2020: Update using 2000-2008 data. Crit Care Med. 2012;40:18–20. doi: 10.1097/CCM.0b013e31822e9ffd. [DOI] [PubMed] [Google Scholar]
- 4.Frengley J.D., Sansone G.R., Shakya K., Kaner R.J. Prolonged mechanical ventilation in 540 seriously ill older adults: Effects of increasing age on clinical outcomes and survival. J Am Geriatr Soc. 2014;62:1–9. doi: 10.1111/jgs.12597. [DOI] [PubMed] [Google Scholar]
- 5.Cox C.E., White D.B., Hough C.L. Effects of a personalized web-based decision aid for surrogate decision makers of patients with prolonged mechanical ventilation. Ann Intern Med. 2019;170:285–287. doi: 10.7326/M18-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lloyd-Owen S.J., Donaldson G.C., Ambrosino N. Patterns of home mechanical ventilation use in Europe: Results from the Eurovent survey. Eur Respir J. 2005;25:1025–1031. doi: 10.1183/09031936.05.00066704. [DOI] [PubMed] [Google Scholar]
- 7.Murphy P., Hart N. Who benefits from home mechanical ventilation? Clin Med. 2009;9:160–163. doi: 10.7861/clinmedicine.9-2-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mandal S., Suh E., Davies M. Provision of home mechanical ventilation and sleep services for England survey. Thorax. 2013;68:880–881. doi: 10.1136/thoraxjnl-2013-203566. [DOI] [PubMed] [Google Scholar]
- 9.Garner D.J., Berlowitz D.J., Douglas J. Home mechanical ventilation in Australia and New Zealand. Eur Respir J. 2013;41:39–45. doi: 10.1183/09031936.00206311. [DOI] [PubMed] [Google Scholar]
- 10.Simonds A.K. Home mechanical ventilation: An overview. Ann Am Thorac Soc. 2016;13:2035–2044. doi: 10.1513/AnnalsATS.201606-454FR. [DOI] [PubMed] [Google Scholar]
- 11.Rose L., McKim D.A., Katz S.L. Home mechanical ventilation in Canada: A national survey. Respir Care. 2015;60:695–704. doi: 10.4187/respcare.03609. [DOI] [PubMed] [Google Scholar]
- 12.Masefield S., Vitacca M., Dreher M. Attitudes and preferences of home mechanical ventilation users from four European countries: An ERS/ELF survey. ERJ Open Res. 2017;3 doi: 10.1183/23120541.00015-2017. 00015-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson J.E., Cox C.E., Hope A.A., Carson S.S. Chronic critical illness. Am J Respir Crit Care Med. 2010;182:446–454. doi: 10.1164/rccm.201002-0210CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kahn J.M., Benson N.M., Appleby D. Long-term acute care hospital utilization after critical illness. JAMA. 2010;303:2253–2259. doi: 10.1001/jama.2010.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Damuth E., Mitchell J., Bartock J. Long-term survival of critically ill patients treated with prolonged mechanical ventilation: A systematic review and meta-analysis. Lancet Respir Med. 2015;3:544–553. doi: 10.1016/S2213-2600(15)00150-2. [DOI] [PubMed] [Google Scholar]
- 16.Unroe M., Kahn J.M., Carson S.S. One-year trajectories of care and resource utilization for recipients of prolonged mechanical ventilation: A cohort study. Ann Intern Med. 2010;153:167–175. doi: 10.1059/0003-4819-153-3-201008030-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frengley J.D., Sansone G.R., Kaner R.J. Chronic comorbid illnesses predict the clinical course of 866 patients requiring prolonged mechanical ventilation in a long-term, acute-care hospital. J Intensive Care Med. 2020;35:745–754. doi: 10.1177/0885066618783175. [DOI] [PubMed] [Google Scholar]
- 18.Huttmann S., Windisch W., Storre J. Invasive home mechanical ventilation: Living conditions and health-related quality of life. Respiration. 2015;89:312–321. doi: 10.1159/000375169. [DOI] [PubMed] [Google Scholar]
- 19.Huttmann S.E., Magnet F.S., Karagiannidis Quality of life and life satisfaction are severely impaired in patients with long-term invasive ventilation following ICU treatment and unsuccessful weaning. Ann Intensive Care. 2018;8:38. doi: 10.1186/s13613-018-0384-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dreyer P.S., Steffensen B.F., Pedersen B.D. Life with home mechanical ventilation for young men with Duchenne muscular dystrophy. J Adv Nurs. 2010;66:753–762. doi: 10.1111/j.1365-2648.2009.05233.x. [DOI] [PubMed] [Google Scholar]
- 21.Ballangrud R., Bogsti W.B., Johansonn I.S. Clients' experiences of living at home with a mechanical ventilator. J Adv Nurs. 2008;65:424–434. doi: 10.1111/j.1365-2648.2008.04907.x. [DOI] [PubMed] [Google Scholar]
- 22.MacIntyre E., Asadi L., Mckim D., Bagshaw S. Clinical outcomes associated with home mechanical ventilation: A systematic review. Can Respir Care. 2016;2016:6547180. doi: 10.1155/2016/6547180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobs J.M., Marcus E.L., Stessman J. Prolonged mechanical ventilation: A comparison of patients treated at home compared to Hospital Long-Term-Care. JAMDA. July 26, 2020 doi: 10.1016/j.jamda.2020.06.038. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Stessman J., Ginsberg G., Hammerman-Rozenberg R. Decreased hospital utilization by older adults attributable to a home hospitalization program. J Am Geriatr Soc. 1996;44:591–598. doi: 10.1111/j.1532-5415.1996.tb01449.x. [DOI] [PubMed] [Google Scholar]
- 25.Stessman J., Hammerman-Rozenberg R., Maaravi Y., Cohen A. Home hospital care. J Am Geriatr Soc. 2000;48:344–345. doi: 10.1111/j.1532-5415.2000.tb02662.x. [DOI] [PubMed] [Google Scholar]
- 26.Maaravi Y., Cohen A., Hammerman-Rozenberg R., Stessman J. Home hospitalization. J Am Med Dir Assoc. 2002;3:114–118. [PubMed] [Google Scholar]
- 27.Jacobs J.M., Hammerman-Rozenberg R., Stessman J. Home hospitalization: 15 years of experience. Ann Intern Med. 2006;144:456. doi: 10.7326/0003-4819-144-6-200603210-00023. [DOI] [PubMed] [Google Scholar]
- 28.Jacobs J.M., Cohen A., Rozengarten O. Closure of a home hospital program: Impact on hospitalization rates. Arch Gerontol Geriatr. 2007;45:179–189. doi: 10.1016/j.archger.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 29.Collin C., Wade D.T., Davies S., Horne V. The Barthel index: A reliability study. Int Disabil Study. 1988;10:61–63. doi: 10.3109/09638288809164103. [DOI] [PubMed] [Google Scholar]
- 30.Lam S.C., Lee D.T., Yu D.S. Establishing cutoff values for the simplified Barthel index in elderly adults in residential care homes. J Am Geriatr Soc. 2014;62:575–577. doi: 10.1111/jgs.12716. [DOI] [PubMed] [Google Scholar]
- 31.Alberta Health Services Edmonton Guidelines for using the revised Edmonton Symptom Assessment System (ESAS-r) 2010. http://www.palliative.org/NewPC/_pdfs/tools/3C7%20ESAS-r%20guidelines%20Aug%2022%202014.pdf Available at:
- 32.Watanabe S.M., Nekolaichuk C., Beaumont C. A multicenter study comparing two numerical versions of the Edmonton Symptom Assessment System in palliative care patients. J Pain Symptom Manage. 2011;41:456–468. doi: 10.1016/j.jpainsymman.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 33.Hui D., Bruera E. The Edmonton Symptom Assessment System 25 years later: Past, present, and future developments. J Pain Symptom Manage. 2017;53:630–633. doi: 10.1016/j.jpainsymman.2016.10.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoyl M.T., Alessi C.A., Harker J.O. Development and testing of a five-item version of the Geriatric Depression Scale. J Am Geriatr Soc. 1999;47:873–878. doi: 10.1111/j.1532-5415.1999.tb03848.x. [DOI] [PubMed] [Google Scholar]
- 35.Hung M.C., Yan Y.H., Fan P.S. Measurement of quality of life using EQ-5D in patients on prolonged mechanical ventilation: comparison of patients, family caregivers, and nurses. Qual Lif Res. 2010;19:721–727. doi: 10.1007/s11136-010-9629-1. [DOI] [PubMed] [Google Scholar]
- 36.Windisch W., Freidel K., Schucher B. The Severe Respiratory Insufficiency (SRI) questionnaire. A specific measure of health-related quality of life in patients receiving home mechanical ventilation. J Clin Epidemiol. 2003;56:752–759. doi: 10.1016/s0895-4356(03)00088-x. [DOI] [PubMed] [Google Scholar]
- 37.Markussen H., Lehmann S., Nilsen R., Natvig G.K. Health-related quality of life as predictor for mortality in patients treated with long-term mechanical ventilation. BMC Pulm Med. 2019;19:13. doi: 10.1186/s12890-018-0768-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walke L.M., Gallo W.T., Tinetti M.E., Fried T.R. The burden of symptoms among community-dwelling older persons with advanced chronic disease. Arch Intern Med. 2004;164:2321–2324. doi: 10.1001/archinte.164.21.2321. [DOI] [PubMed] [Google Scholar]
- 39.Kako J., Kobayashi M., Kanno Y. The optimal cutoff point for expressing revised Edmonton Symptom Assessment System scores as binary data indicating the presence or absence of symptoms. Am J Hosp Palliat Med. 2018;35:1390–1393. doi: 10.1177/1049909118775660. [DOI] [PubMed] [Google Scholar]
- 40.Dhiliwal S., Salins N., Deodhar J. Pilot testing of triage coding system in home-based palliative care using Edmonton Symptom Assessment Scale. Indian J Palliative Care. 2016;22:19–24. doi: 10.4103/0973-1075.173943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jacobs J.M., Maaravi Y., Cohen A. The changing profile of health and function from age 70-85. Gerontology. 2012;58:313–321. doi: 10.1159/000335238. [DOI] [PubMed] [Google Scholar]
- 42.Bentur N., Heymann A.D. Depressive symptoms and use of health services among older adults in Israel. Isr J Health Policy Res. 2020;9:15. doi: 10.1186/s13584-020-00374-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blazer D.G. Depression in late life: Review and commentary. J Gerontol A Biol Sci Med Sci. 2003;58:249–265. doi: 10.1093/gerona/58.3.m249. [DOI] [PubMed] [Google Scholar]
- 44.Jubran A., Grant B., Duffner L. Long-term outcome after prolonged mechanical ventilation. Along-term acute-care hospital study. Am J Respir Crit Care Med. 2019;199:1508–1516. doi: 10.1164/rccm.201806-1131OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marchese S., Coco D.L., Coco A.L. Outcome and attitudes toward home tracheostomy ventilation of consecutive patients: A 10-year experience. Respir Med. 2008;102:430–436. doi: 10.1016/j.rmed.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 46.Narayanaswami P., Bertorini T.E., Pourmand R., Horner L.H. Long-term tracheostomy ventilation in neuromuscular diseases: Patient acceptance and quality of life. Neurorehabil Neural Repair. 2000;14:135–139. doi: 10.1177/154596830001400206. [DOI] [PubMed] [Google Scholar]
- 47.Law A.C., Walkey A.J. Long-term outcomes after prolonged mechanical ventilation: What of those cast away? Am J Respir Crit Care Med. 2019;199:1579–1580. doi: 10.1164/rccm.201901-0210LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jubran A., Grant B.J.B., Tobin M.J. Reply to Law and Walkey: Long-term outcomes after prolonged mechanical ventilation: What of those cast away? Am J Respir Crit Care Med. 2019;199:1580–1581. doi: 10.1164/rccm.201902-0368LE. [DOI] [PMC free article] [PubMed] [Google Scholar]