Figure 4.
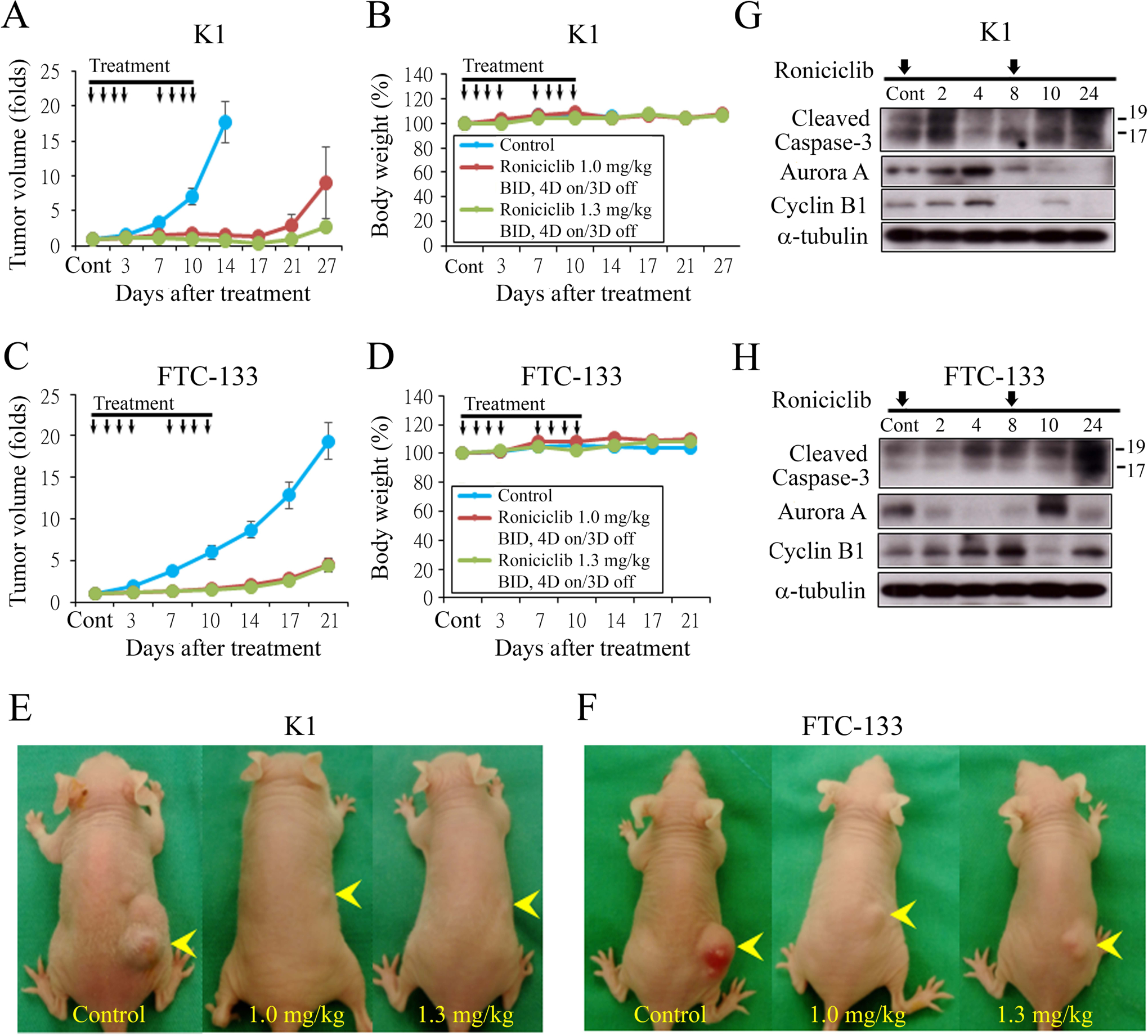
Roniciclib inhibits subcutaneous xenograft growth of two well-differentiated thyroid cancer models. (A) The therapeutic efficacy of roniciclib for papillary thyroid cancer was evaluated in mice bearing K1 flank tumors. Serial oral gavage of low-dose (1.0 mg/kg) and high-dose (1.3 mg/kg) roniciclib significantly repressed K1 tumor growth by day 3 when compared with control mice, and the effect persisted through day 14. (B) Serial treatment of low-dose (1.0 mg/kg) and high-dose (1.3 mg/kg) roniciclib did not significantly decrease body weight when compared with control mice. (C) The therapeutic efficacy of roniciclib for follicular thyroid cancer was evaluated in mice bearing FTC-133 flank tumors. Serial oral gavage of low-dose (1.0 mg/kg) and high-dose (1.3 mg/kg) roniciclib significantly repressed FTC-133 tumor growth by day 3 when compared with control mice, and the effect persisted through day 21. (D) Serial treatment of high-dose roniciclib (1.3 mg/kg) slightly, but significantly induced body weight loss on day 10 when compared with control mice. This effect was not observed in mice treated with low-dose (1.0 mg/kg) roniciclib. K1 (E) and FTC-133 (F) xenograft tumors (arrowhead) were photographed on day 15. The molecular effects of roniciclib (1.3 mg/kg) treatment were evaluated in K1 (G) and FTC-133 tumors (H) using Western blot analysis. Arrow, roniciclib or placebo treatment.
