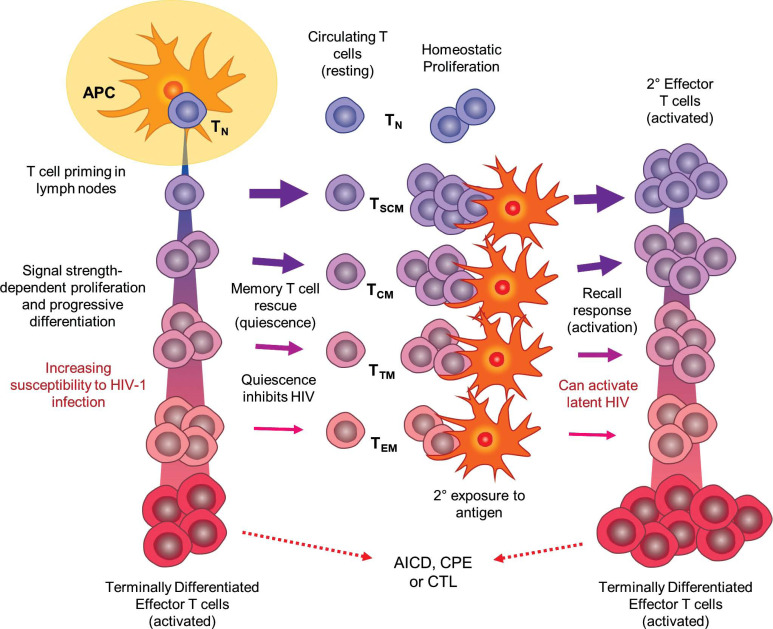
Fig. (1).
Depiction of the DP/PD model of CD4+ T cell differentiation and immune response as a framework for HIV-1 infection, latency, and re-activation (see text for details). Upon initial antigen exposure, naïve T cells (TN) are primed by antigen-presenting cells (APCs) in secondary lymphoid organs. Depending on the strength and quality of stimulatory signals, CD4+ T cells progress along an activation and differentiation pathway, losing naïve and acquiring effector properties. It is postulated that less differentiated cells are rescued into memory with greater frequency, while highly differentiated effectors are more susceptible to HIV-1 infection and support virus replication. All terminally differentiated and most other effector cells are not rescued into memory, but are instead subject to programmed, activation-induced cell death (AICD), are killed by cytotoxic T-lymphcytes (CTL), or if infected with HIV-1, are killed by the cytopathic effects of active virus. Latency is established when infected effector cells are rescued into memory T cells in the resting state after the stimulating antigen is removed. Differentiation of memory cell subsets (TSCM, TCM, TTM, TEM) parallels effector cell differentiation, with less specialized cells tending to be longer-lived and having a greater capacity for homeostatic proliferation. Response to subsequent antigen exposure is faster and more potent as memory cells re-enter the effector differentiation pathway; however, this process may also cause quiescent HIV-1 to emerge from latency. As with initial exposure, some secondary effector cells are rescued into memory after the antigen has been removed, thus perpetuating the cycle of T-cell maintenance and response, and potentially HIV-1 latency.