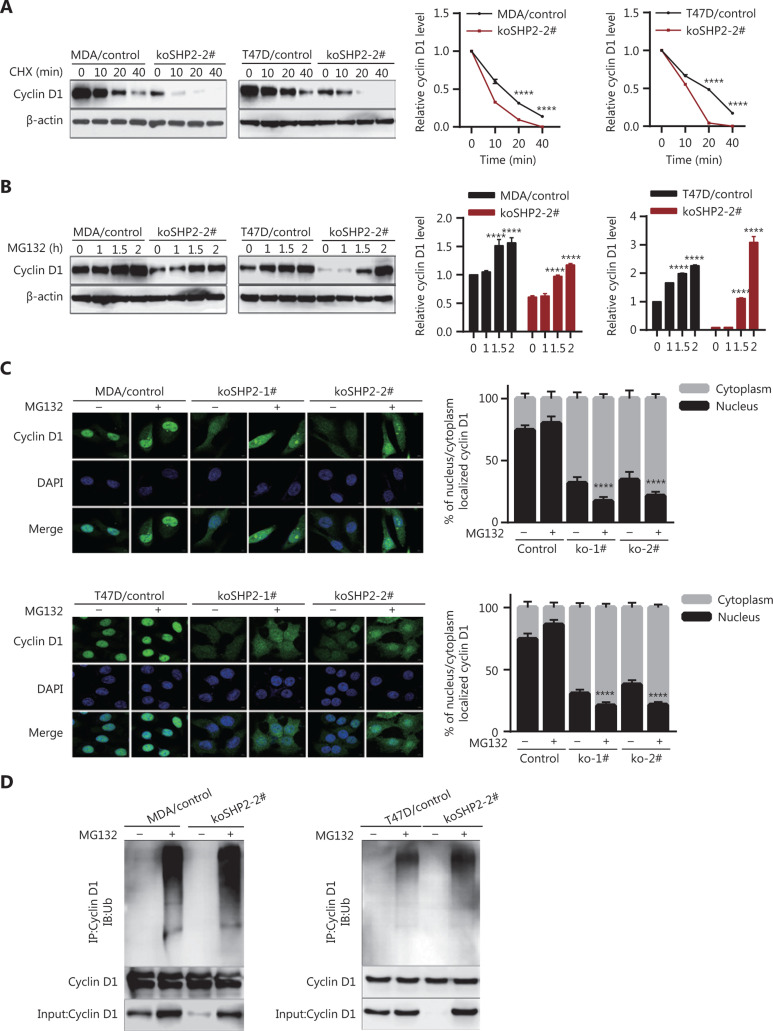
Figure 4.
SHP2 knockout promotes Cyclin D1 degradation through the ubiquitin–proteasome pathway. (A) The half-life of Cyclin D1 in SHP2 knockout cells was significantly shorter than that in control cells. The cells were treated with cycloheximide for the indicated times, and the expression of Cyclin D1 was analyzed by western blotting. (****P < 0.0001). (B) The expression level of Cyclin D1 in SHP2 knockout cells was restored by MG132 treatment. The cells were treated with 10 μM of MG132 for the indicated times, and the expression of Cyclin D1 was analyzed by western blotting (****P < 0.0001). (C) Immunofluorescence staining showed that MG132 treatment resulted in an elevation of Cyclin D1 in SHP2 deleted cells. Cyclin D1 was mainly localized in the nuclei in control cells, whereas the increased Cyclin D1 after MG132 treatment in SHP2 deleted cells was mainly localized in the cytoplasm. The quantification of Cyclin D1 nucleus/cytoplasm ratio is shown in the right panel (****P < 0.0001). (D) Significantly greater ubiquitinated Cyclin D1 was observed in SHP2 knockout cells than in control cells. The control and SHP2 knockout cells were treated with MG132 or left untreated, and were then lysed and immunoprecipitated with anti-Cyclin D1. The enriched proteins were analyzed by western blotting with anti-Cyclin D1 and anti-ubiquitin antibodies.