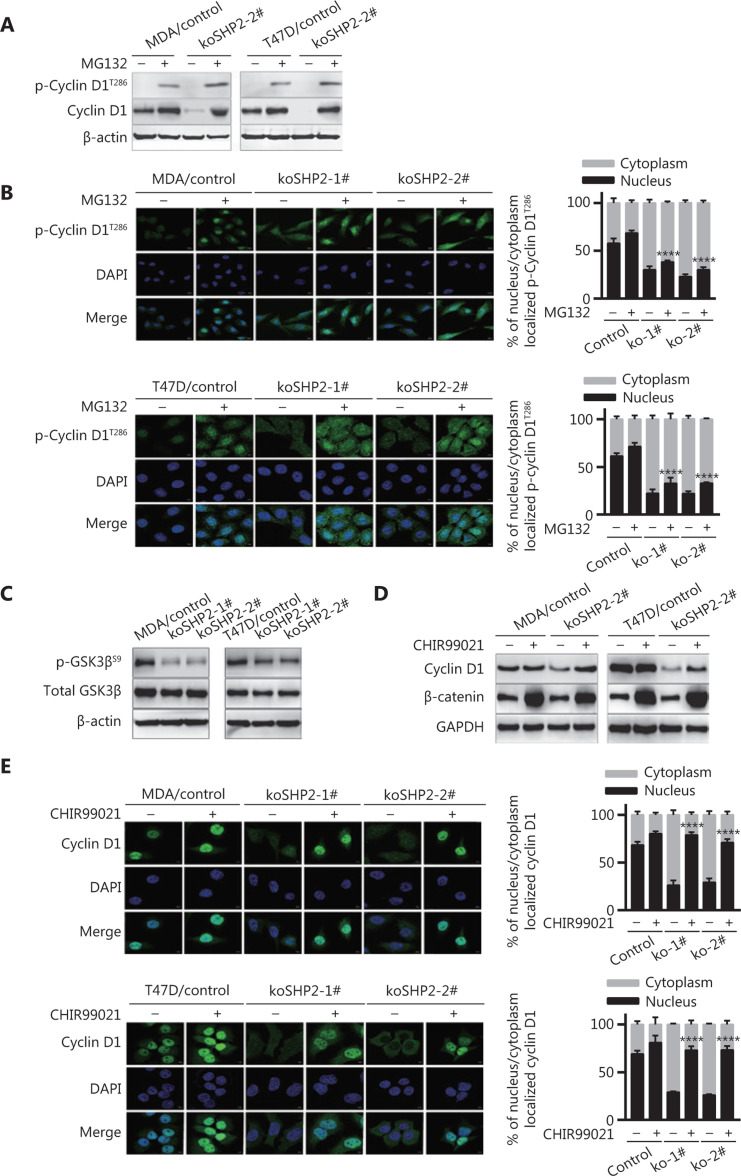
Figure 5.
GSK3β-induced T286 phosphorylation of Cyclin D1 is responsible for Cyclin D1 proteasomal degradation. (A) SHP2 knockout increased the protein levels of phosphorylated Cyclin D1 at T286 in the presence of MG132 (10 μM). (B) Immunofluorescence staining showed that MG132 treatment considerably increased the level of phosphorylated Cyclin D1 (T286). Cells were treated with 10 μM of MG132 for 6 h, fixed, and stained with anti-p-Cyclin D1 (T286) antibodies. The quantification of the p-Cyclin D1 (T286) nucleus/cytoplasm ratio is shown in the right panel. Statistical analysis was carried out with one-way ANOVA (****P < 0.0001). (C) Western blot analysis of the expression of total and phosphorylated GSK3β (Ser9) in cell lysates from the control and SHP2 knockout cells. (D) The protein level of Cyclin D1 in SHP2 deleted cells recovered after treatment with the GSK3β inhibitor CHIR99021. Control and SHP2 deleted cells were treated with 20 μM of CHIR99021 for 6 h or left untreated, and the expression of Cyclin D1 was analyzed by western blotting. (E) Immunofluorescence staining showed that CHIR99021 treatment significantly increased the nuclear expression of Cyclin D1 in the control and SHP2 deleted cells. The quantification of the Cyclin D1 nucleus/cytoplasm ratio is shown in the right panel (****P < 0.0001).