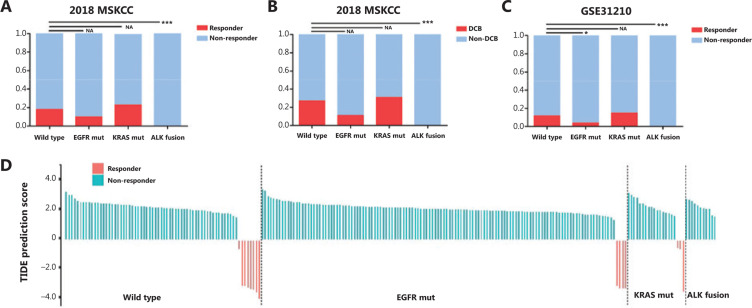
Figure 1.
Correlation between the efficacy of anti-PD-1/PD-L1 immunotherapy and classic driver oncogene mutations in non-small cell lung cancer (NSCLC) patients. (A and B) Box plots evaluating objective response rate (A) and durable clinical benefit (progression-free survival > 6 months) (B) of NSCLC patients harboring EGFR mutations, KRAS mutations, and ALK fusions after initiation of PD-1/PD-L1 blockade treatment, in the 2018 MSKCC database. (C) A box plot evaluating the objective response rate of NSCLC patients, using TIDE prediction scores in the GSE31210 database. (D) A waterfall plot of TIDE prediction scores across 226 NSCLC tumors in the GSE31210 database. Red indicates a tumor that responded to therapy. Blue indicates non-responders. Tumors were divided into 4 categories based on the molecular genotype of NSCLC. In each category, we sorted tumors in descending order according to their TIDE prediction scores. *P < 0.05; **P < 0.01; ***P < 0.001. EGFR mut, EGFR mutation; KRAS mut, KRAS mutation; DCB, durable clinical benefit; Non-DCB, no durable clinical benefit.