Abstract
Small-cell lung cancer (SCLC) is a poorly differentiated neuroendocrine neoplasm with inadequate therapeutic options. Fasudil is a Rho-associated protein kinase 1 and 2 (ROCK1/2) inhibitor whose clinical indications remain limited in cardiocerebrovascular diseases. This study aimed to report a possible implication of Fasudil for SCLC. The expression and prognostic value of ROCK1/2 were investigated immunohistochemically in surgical specimens. The positive rates of ROCK1 (77/113, 68.1%) and ROCK2 (94/113, 83.2%) were distinctly higher in SCLC than in other lung neuroendocrine tumors. The high expression level of ROCK1 was related to the poor long-term survival of patients, especially in the classic SCLC subtype. In vitro, SCLC cell line treated with Fasudil exhibited synapse-like morphologic change, accompanied by a reduction in the expression levels of c-myc and cyclin D1. Cell cycle arrest was further demonstrated, accompanied by sensitivity to starvation-induced apoptosis, indicating tumor maturation. In addition, RNA-seq identified hundreds of differentially expressed genes involved in the positive regulation of neuron differentiation, stem cell differentiation, cell development, and nervous system development. Finally, Fasudil inhibited SCLC growth, promoted structural maturity, and induced apoptosis in BALB/c nude mice xenograft model. In conclusion, these results indicated a potential and novel application of Fasudil for SCLC treatment.
Keywords: Apoptosis, Fasudil, Rho-associated protein kinase, small-cell lung cancer, tumor maturation
Introduction
Small-cell lung cancer (SCLC), an extremely malignant lung neuroendocrine neoplasm, accounts for about 15% of lung cancers. It is characterized by rapid tumor growth, early metastasis, and acquired chemoresistance. Generally, the median survival time of patients with SCLC is 7-10 months. The 5-year survival rate is approximately 20%-25% for limited-stage SCLC, but barely 1%-2% for extensive-stage SCLC [1]. Surgery can cure only a small minority of patients with localized limited-stage SCLC. A large majority of patients with SCLC have metastasis at the first time of diagnosis.
SCLC is initially sensitive to chemotherapy, but later acquires chemoresistance. Therapeutic options for SCLC have developed slowly over the past years. For several decades, the standard first-line chemotherapy regimen for SCLC has been a platinum agent (cisplatin or carboplatin) and etoposide [1,2]. Recently, atezolizumab [an anti- programmed cell death 1 ligand 1 (PDL1) antibody] has improved median survival from 10.3 to 12.3 months in the first-line therapy for advanced SCLC [3]. Besides, the US Food and Drug Administration has approved topotecan [4] (a topoisomerase I poison) and nivolumab [5] (a PD1 antagonist for second- or third-line use) in recurrent SCLC. However, only less than 20% patients could reap benefits durable benefits from these novel drugs Besides, over the past three decades, although many clinical trials evaluated new chemotherapy schemes and various biological agents, no significant improvement has been found in the overall survival (OS) time of patients with SCLC. Thus, obviously, more effective therapies are needed for SCLC.
The Rho-associated coiled-coil-containing protein serine/threonine kinases (ROCK) include two mammalian homologs, ROCK1 and ROCK2 (referred as ROCK1/2 hereafter). ROCK1/2 are effectors of the Rho subfamily of small guanosine triphosphatases (GTPases) [6]. The ROCK signaling pathway suppresses repair after nerve injury [7]. On the contrary, ROCK activation enhances actomyosin contractility [8], accelerates retraction forces at the axon terminal [9], stresses fiber formation [10], and thus limits nerve regeneration. Pharmacological ROCK inhibition with Fasudil, Dimethylfasudil, or Y-27632 offsets neurite retraction and contributes to neurite outgrowth [11,12]. Inhibition of ROCK signaling also promotes axonal elongation in dorsal root ganglia [13]. Besides, in the rat optic nerve crush model, a simultaneous local administration of the ROCK inhibitor Y-27632 in a dose-dependent manner leads to axonal regeneration in retinal ganglion cells [14]. Different studies of rodent spinal cord injury have demonstrated similar results, in which animals were locally treated with Y-27632 or Fasudil after hemisection [15]. Then again, ROCK signaling plays an important role in the maturation of oligodendrocytes and formation of myelin sheaths [16,17]. Remyelination prevents axonal degeneration and is a key process for successful treatment after nerve injury. However, constantly elevated activation of the RhoA-ROCK pathway inhibits oligodendrocyte precursor cell differentiation, causing failure in remyelination [17].
Recently, Fasudil was considered to be the only ROCK inhibitor approved for human clinical therapy, which has been used for cerebral vasospasm following subarachnoid hemorrhage since 1995. ROCK inhibition by Fasudil was recently reported to induce terminal adipocyte differentiation and suppress tumorigenesis in chemoresistant osteosarcoma cells [18]. SCLC is a poorly differentiated or undifferentiated neuroendocrine carcinoma. Given the role of ROCK1/2 inhibitor in neuronal regeneration and oligodendrocyte precursor cell differentiation, a hypothesis was put forward that Fasudil might be an effective inducer for SCLC maturation.
Materials and methods
Specimens of patients
Tumor specimens and adjacent normal lung tissues were obtained from surgically resected specimens. Tumors diagnosed from 2012 to 2018 were retrieved with informed consent from archival sources at the Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. All cases were reviewed by three experienced pathologists independently. Only those cases that complied with the latest World Health Organization classification of tumors were included. This study was approved by the ethical committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology.
Immunohistochemical analysis
The staining was conducted on tissue array or slices of cell block and primary antibodies including anti-ROCK1 rabbit monoclonal antibody (ab45171, 1:200; Abcam, MA, USA), anti-ROCK2 rabbit polyclonal antibody (ab71598, 1:200; Abcam), anti-c-myc antibody (ZA-0555, 1:1000; ZSGB-BIO, China), anti-cyclin D1 antibody (TA804673, 1:1000; ZSGB-BIO), anti-Achaete-Scute Complex-Like 1 (ASCL1) antibody (1:1000, ab211327; Abcam). Sections were deparaffinized and hydrated, and the endogenous peroxidase was blocked. Antigen retrieval was performed using the Dako Target Retrieval Solution, High pH (Dako Ominis, Agilent Technologies, CA, USA), in a PTLink set at 98°C for 25 min. Then, the slides were incubated with the primary antibody for 60 min at room temperature. Immunostaining was achieved using an enzyme-conjugated polymer complex (Dako K8002, Agilent Technologies) adapted for an autostainer (Dako Autostainer Link 48, Agilent Technologies). Adjacent normal lung tissues were used as a negative control (NC). Immunohistochemical reactivity was scored by three pathologists (Zitian Huo, Yaqi Duan, and Guoping Wang) independently.
Cell line and reagents
Human SCLC cell line, NCI-H1339 cell line, was donated by Dr. Hui Pan from Sun Yat-sen University Cancer Center and validated by short tandem repeat profiling as well as immunohistochemistry (IHC) for CK7 and Napsin A to exclude lung adenocarcinoma contamination (Figures S1, S2A). The cells were cultured in Dulbecco’s Modified Eagle’s medium/Ham’s F12 medium supplemented with 10% fetal bovine serum. Standard Fasudil hydrochloride (YZ-100614) was purchased from Solarbio Life Science Company, China.
Cell block preparation
The NCI-H1339 cells were divided into NC group or Fasudil stimulation group. After stimulation with 100 μg/mL Fasudil for 48 h, the cells of both groups were collected using a rubber head dropper inserted into a 10-cm centrifuge tube upside down; the cells were then concentrated and fixed in formalin overnight. The dropper was cut to preserve only the bottom containing cell precipitate. This bottom was packaged into a filter paper, placed in a full-automatic tissue hydroextractor, and finally embedded to form a cell block.
Cell viability assay
The cells were seeded into six-well culture plates at a density of 5 × 104 cells per well. After 12 h, the cells were treated with different doses of Fasudil (0, 1, 5, 10, 25, 50, 100, and 200 μg/mL) or treated with 100 μg/mL of Fasudil for different time points (0, 12, 24, and 48 h). At the indicated time, the Cell Counting Kit-8 (CCK-8; CK04-100, Dojindo, Kumamoto Prefecture, Kyushu, Japan) assay was used to conduct cell viability. The absorbance at 450 nm was read using a multimode microplate reader (BioTek Instruments, Inc., VT, USA). A nonlinear regression (curve fit) analysis was conducted on GraphPad Prism 7.0 as log (inhibitor) versus normalized response to calculate the half-inhibitory concentration (IC50) of Fasudil inhibition on SCLC cells.
Cell cycle analysis and Annexin V-FITC/PI assay for apoptosis
The NCI-H1339 cells were seeded into six-well plates at a density of 2 × 105 cells per well. The cells were treated with 50 or 100 μg/mL of Fasudil for 24 or 48 h at 37°C. For cell cycle assays, the cells were harvested and fixed in 70% cold ethanol overnight at 4°C. After washing with phosphate buffer saline (PBS), the cells were incubated with RNase A for 30 min at 37°, followed by staining with propidium iodide for 30 min in the dark.
For apoptosis assays, the cells were divided into five groups as follows: group 1 had no treatment for 96 h and served as an NC; group 2 was treated with Fasudil (100 µg/mL) for 48 h, and then the culture medium was exchanged using a fresh medium; group 3 was treated with Fasudil for 96 h without medium exchange; group 4 was cultured for 48 h first, and then the medium was exchanged together with Fasudil stimulation for another 48 h; group 5 was cultured for 48 h first, but stimulated with Fasudil for another 48 h with the exchanged culture medium. In the end, the cells were detected using an Annexin V-Fluorescein Isothiocyanate/Propidium Iodide (FITC/PI) Apoptosis Detection Kit (556,547; BD Biosciences, CA, USA). All samples for cell cycle assays and apoptosis were analyzed using a FACScan flow cytometer (BD Biosciences).
RNA-seq and time-series analysis
The NCI-H1339 cells were treated with 100 μg/mL Fasudil for 12 h as group A and for 24 h as group B. The cells without any stimulation served as the NC group.
The RNA-seq was conducted by the Bioacme company in Wuhan, China. Total RNA was extracted using a TRIzol reagent (Invitrogen, Shanghai, China) following the the manufacturer’s protocols. The availability of experimental samples was ensured by testing the concentration, purity, and integrity of total RNA. After the samples were tested to be qualified, mRNA purification and chain-specific library preparation were carried out. The prepared library was subjected to library quality inspection. After the library passed the quality inspection, it was sequenced on the computer. After the RNA sample passed the test, the eukaryotic mRNA (in the case of prokaryote, the mRNA was enriched by removing rRNA through the kit) was enriched using magnetic beads with oligo (DT). After that, a fragment buffer was added to break the mRNA into short segments. mRNA was used as a template to synthesize a single-stranded cDNA with six base random primers. Then, buffer, dNTPs, DNA polymer I, and RNaseH were added to synthesize double-stranded cDNA. Then, the double-stranded cDNA was purified with AMPure XP beads. The purified double-stranded cDNA was first repaired with A-tail and connected with the sequencing connector; then, the fragment size was selected using AMPure XP beads. After that, the second strand of U-containing cDNA was degraded with user enzyme so that the final sequencing information came from the first strand of cDNA, thus preserving the strand orientation of mRNA. Finally, the polymerase chain reaction (PCR) amplification was carried out, and the PCR products were purified with AMPure XP beads to obtain the chain-specific cDNA library. After the construction of the library, qubit 3.0 was used for preliminary quantification, and the library was diluted to 1 ng/UL. Then, Qsep100 was used to detect the insert size of the library. After the insert size met the expectation, qPCR was used to accurately quantify the effective concentration of the library (the effective concentration of the library was >2 nm), so as to ensure the quality of the library. After the library passed the inspection, different libraries were pooled according to the requirements of effective concentration and target offline data volume, following which HiSeq sequencing was carried out.
In RNA-seq, differentially expressed genes between the samples were selected based on two levels: fold change (|log2(Fold change)|>1) and significance (P < 0.05). Furthermore, time-series analysis was conducted using the Mfuzz package as instruction. The related Gene Ontology (GO) analysis and cluster analysis of gene expression were then conducted in each cluster. The analysis of the sequencing data was carried out using the BMKCloud platform (http://www.biocloud.net/). The information on related pathways referred to the Kyoto Encyclopedia of Genes and Genomes pathways (https://www.kegg.jp).
RNA isolation and quantitative real-time PCR
Total RNA was isolated from cells using a TRIzol reagent and then reverse-transcribed (RT) into cDNA using a RevertAid cDNA Synthesis Kit (RR047A, TaKaRa, Tokyo, Japan). The RT-PCR was performed using the primers in Table 1. Then, the cDNA levels were amplified using an ABI Prism 7900HT platform (Applied Biosystems, CA, USA) after adding SYBR Green PCR Master Mix (04913850001, Roche, Basel, Switzerland). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control. The relative expression levels were determined from cycle threshold values and calculated as fold change compared with controls.
Table 1.
Sequences of primers for qRT-PCR
| Gene | Primer sequence (5’ to 3’) |
|---|---|
| PLK5 | |
| Forward | CTCCCTGTCTGCGAAAGAGG |
| Reverse | CCGTCCAAGAGCTGGTAGC |
| SLIT2 | |
| Forward | AGCTTAGACGAATTGACCTGAGC |
| Reverse | CCGAAGGCAGTTTATCTTGTTGG |
| CDK6 | |
| Forward | CCAGATGGCTCTAACCTCAGT |
| Reverse | AACTTCCACGAAAAAGAGGCTT |
| CDK1 | |
| Forward | AAACTACAGGTCAAGTGGTAGCC |
| Reverse | TCCTGCATAAGCACATCCTGA |
| CDKN1C | |
| Forward | GCGGCGATCAAGAAGCTGT |
| Reverse | GCTTGGCGAAGAAATCGGAGA |
| CDKN2A | |
| Forward | GGGTTTTCGTGGTTCACATCC |
| Reverse | CTAGACGCTGGCTCCTCAGTA |
| NGFR | |
| Forward | CCTACGGCTACTACCAGGATG |
| Reverse | CACACGGTGTTCTGCTTGT |
| Netrin 1 | |
| Forward | TGCAAGCCCTTCCACTACG |
| Reverse | TGTTGTGGCGACAGTTGAGG |
| Neurocan | |
| Forward | CAACGCCACGCTACTTCTG |
| Reverse | ATCGGTAGTGGAACACAACAC |
| Neurofilament heavy | |
| Forward | CCGTCATCAGGCCGACATT |
| Reverse | GTTTTCTGTAAGCGGCTATCTCT |
| Neurofilament medium | |
| Forward | GCTCGTCATTTGCGCGAATAC |
| Reverse | TTTCTGTACGCAGCGATTTCTAT |
| Neurofilament light | |
| Forward | CCGTGGAGATGGACGTGAC |
| Reverse | AACCATTCCTCAGCGTTCTGC |
| Neurogenin 2 | |
| Forward | AGGAAGAGGACGTGTTAGTGC |
| Reverse | GCAATCGTGTACCAGACCCAG |
| Neuronal PAS domain protein 1 | |
| Forward | CTCAGCGTCACCTACCTCC |
| Reverse | GGCGAACACAAAGCCATCC |
| SOX11 | |
| Forward | AGCAAGAAATGCGGCAAGC |
| Reverse | ATCCAGAAACACGCACTTGAC |
| GAPDH | |
| Forward | GGAGCGAGATCCCTCCAAAAT |
| Reverse | GGCTGTTGTCATACTTCTCATGG |
Note: CDK1, cyclin-dependent kinase 1; CDK6, cyclin-dependent kinase 6; CDKN1C, cyclin-dependent kinase inhibitor 1C; CDKN2A, cyclin-dependent kinase inhibitor 2A; NGFR, nerve growth factor receptor; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; PLK5, polo-like kinase 5; SLIT2, Slit homolog 2 protein; SOX11, SRY-box 11.
Western blot analysis
The cell pellets were collected, and protein samples were prepared by lysing cells using the RIPA lysis buffer (R0278, Sigma-Aldrich) supplemented with phosphatase and protease inhibitor cocktails (Roche). The total proteins (20-50 μg) were loaded on a 10% SDS-PAGE gel and transferred to a nitrocellulose membrane (Millipore, MA, USA). After blocking for 1 h with 5% bovine serum albumin dissolved in TBST, the membranes were incubated overnight at 4°C with the following primary antibodies: anti-ASCL1 antibody (1:1000, ab211327; Abcam), anti-SRY-related HMG-box 11 (SOX11) antibody (1:1000, ab170916; Abcam), anti-CDK4 antibody (1:1000, ab108357; Abcam), anti-CDK6 (1:1000, ab124821; Abcam), anti-Polo-like kinase 5 (anti-PLK5) antibody (1:1000, ab174755; Abcam), anti-Slit homolog 2 protein (anti-SLIT2) (1:1000, ab134166; Abcam), and anti-beta-actin antibody (1:2000, ab115777; Abcam). After being incubated with the corresponding horseradish peroxidase-labeled secondary antibody for 1 h at room temperature, protein signals were visualized using the Super-Signal West Femto Maximum Sensitivity Substrate (34,095; Thermo Fisher Scientific, MA, USA).
In vivo tumorigenesis
The Animal Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, approved all animal studies. BALB/c athymic nude mice (4-6 weeks) were obtained from the Huafukang Biotechnology Co. Ltd. (Beijing, China). For tumorigenesis, approximately 5 × 106 cells were injected at the middle point of the right elbow and neck subcutaneously. After 2 weeks, when the tumor diameter reached approximately 5 mm, the tumor-bearing mice were randomized into two groups (five per group) and assigned to intraperitoneally receive vehicle (PBS) or Fasudil (50 mg/kg) daily for 14 days. The dose was determined according to a previous study [18]. At the endpoint, the tumors were surgically removed, weighed, and photographed. The tumor volume was calculated as “volume = width2 × length/2”. Tumors were fixed with 10% formaldehyde, embedded in paraffin, and cut into 4-μm sections. Hematoxylin and eosin (HE) staining was conducted following the manufacturer’s protocol (BASO, BA4094, China). Modified Gordon-Sweets Reticulin Stain Kit (G3525, Solarbio) was used following the manufacturer’s protocol n. In Situ Cell Apoptosis Detection Kit II (AP) (MK1017, BOSTER, China) was used for TUNEL assay following the manufacturer’s protocol; the percentage of positive cells was counted to evaluate the apoptosis level.
Statistical analysis
All the experiments were conducted in triplicate. Statistical analysis was performed using the GraphPad Prism 7.0 software. OS was analyzed using the Kaplan-Meier method and compared using the log-rank test and Gehan-Breslow-Wilcoxon test. Data were analyzed using the two samples Student t test for comparison between two groups, which was used in the evaluation of all experiments above except survival analysis. Data were presented as mean ± standard error of mean (SEM). A P value less than 0.05 was considered to be significant.
Results
Expression of ROCK1/2 in lung neuroendocrine tumors and prognostic value in SCLC
The expression of ROCK1/2 was examined in 17 typical carcinomas (TCs), 23 atypical carcinomas (ACs), 35 large-cell neuroendocrine carcinomas (LCNECs), and 113 SCLC samples using IHC. ROCK1 was negative in all TC, AC, and LCNEC samples (Figure 1A), but was positive in 77/113 (68.1%) SCLC samples (Table 2), with 24/113 (21.2%) weak, 17/113 (15.0%) moderate, and 34/113 (30.1%) strong cases. Figure 1B shows the representative HE staining of SCLC, positive IHC staining of ASCL1 in SCLC, and ROCK1/2 IHC staining at different levels. Figure S2C provides the representative IHC images of ROCK1, ROCK2, and ASCL1 negative cases. The weak staining and negative cases were classified into the low-expression group in later survival analysis. Retrospective follow-up information was accessible in 69 patients with SCLC. Of these, ROCK1 was positive in 57/69 (82.6%) samples, including 18/69 (26.1%) weak, 12/69 (17.4%) moderate, and 29/69 (42.0%) strong cases. In Kaplan-Meier analysis, the Gehan-Breslow-Wilcoxon test suggested that patients with the low expression of ROCK1 had better long-term survival (Figure 1C). In classic (ASCL1-positive) SCLC subtype (54/69, 78.3%), both Gehan-Breslow-Wilcoxon and log-rank test reached a statistically significant level, suggesting that ROCK1-negative patients had better OS in both short and long term (Figure 1D). On the contrary, ROCK2 was negative in all AC and LCNEC samples, but was positive in 14/17 (82.4%) TC and 94/113 (83.2%) SCLC samples (Table 2). The expression of ROCK2 did not correlate with the OS time of patients with SCLC (Figure S2).
Figure 1.
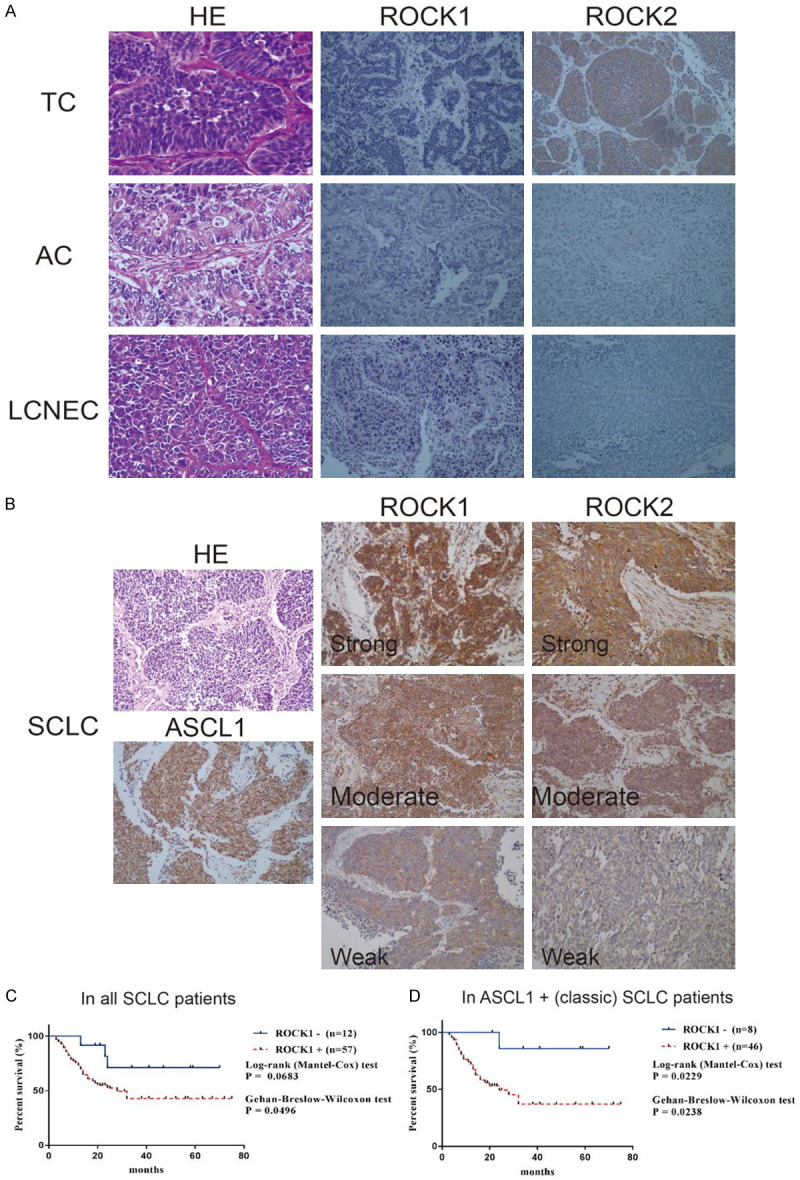
Representative staining images and Kaplan-Meier analysis. (A) Representative HE staining (magnification × 400) and ROCK1/2 IHC staining for TC, AC, and LCNEC (magnification × 200) (B) HE staining of SCLC, positive IHC staining of ASCL1 in SCLC, and ROCK1/2 IHC staining image of strong, moderate, and weak cases (magnification × 200). (C, D) Kaplan-Meier analysis of ROCK1 in all 69 patients with SCLC (C) and in patients with ASCL1-positive (classic subtype) SCLC (D) indicated high expression of ROCK1 was related to poorer overall survival time.
Table 2.
Expression of ROCK1/2 in lung neuroendocrine tumors
| ROCK1 | ROCK2 | |
|---|---|---|
| TC | 0/17, 0% | 14/17, 82.4% |
| AC | 0/23, 0% | 0/23, 0% |
| LCNEC | 0/35, 0% | 0/35, 0% |
| SCLC | 77/113, 68.1% | 94/113, 83.2% |
Effect of Fasudil on SCLC cells in vitro
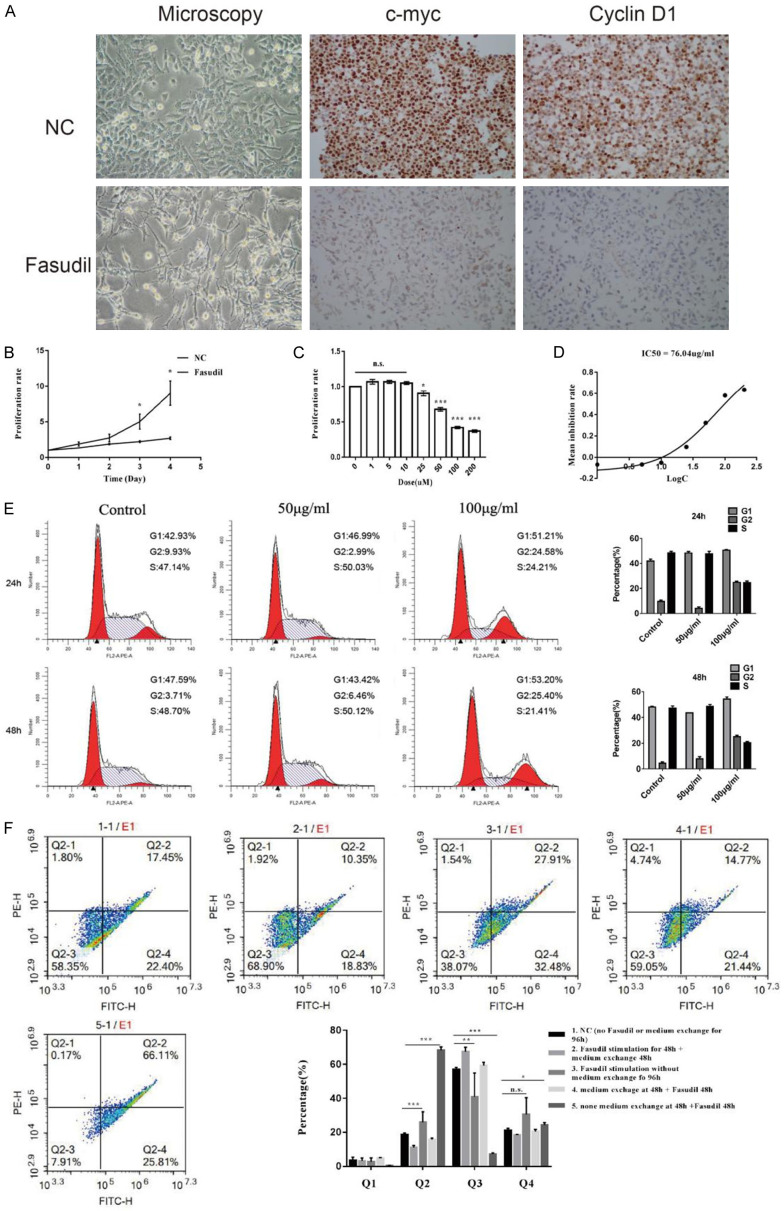
Figure 2A shows that cells distinctly gave rise to synaptoid-like cell structures after stimulation with 100 μg/mL Fasudil for 48 h, which was accompanied by a large reduction in the expression levels of c-myc and cyclin D1. Besides, the proliferation ability was investigated by the cell growth curve and CCK-8 detection. The cell growth was inhibited when treated with 100 μg/mL Fasudil (Figure 2B) for 1-4 days or when treated with a concentration gradient (Figure 2C). Subsequently, the IC50 was measured to be 76.04 μg/mL (Figure 2D).
Figure 2.
A. Microscopic morphology showed cells distinctly gave rise to synaptoid-like cell structures after Fasudil stimulation, which was accompanied by down-regulation of c-myc and cyclin D1 expression (magnification × 200). B. Cell growth curve showed inhibition of cell proliferation after Fasudil stimulation in time gradient. C. Cell proliferation was also inhibited in dose gradient. D. IC50 was measured to be 76.04 μg/mL. E. Cell cycle arrest was detected after stimulation of 100 μg/mL Fasudil for 24 and 48 h. F. Annexin V-FITC/PI assay showed Fasudil promoted SCLC cell apoptosis, which effect was especially obvious in the starved cells and could be blocked or reversed by relief of starvation. Data were presented as mean ± SEM, n = 3; *P < 0.05, **P < 0.01, and ***P < 0.001.
As shown by the cell cycle analysis, after stimulation with 100 μg/mL of Fasudil for 24 and 48 h, the significantly reduced in the S phase, slightly accumulated in the G0/G1 phase, and obviously accumulated in the G2/M phase (Figure 2E). Moreover, stimulation with 100 μg/mL Fasudil promoted SCLC cell apoptosis (Figure 2F, group 1 vs group 3). This effect was especially obvious in the starved cells (group 5 vs group 3) and could be blocked or reversed by relief of starvation (group 5 vs group 4 and group 2 vs group 3).
Transcript profile changes after Fasudil stimulation in vitro
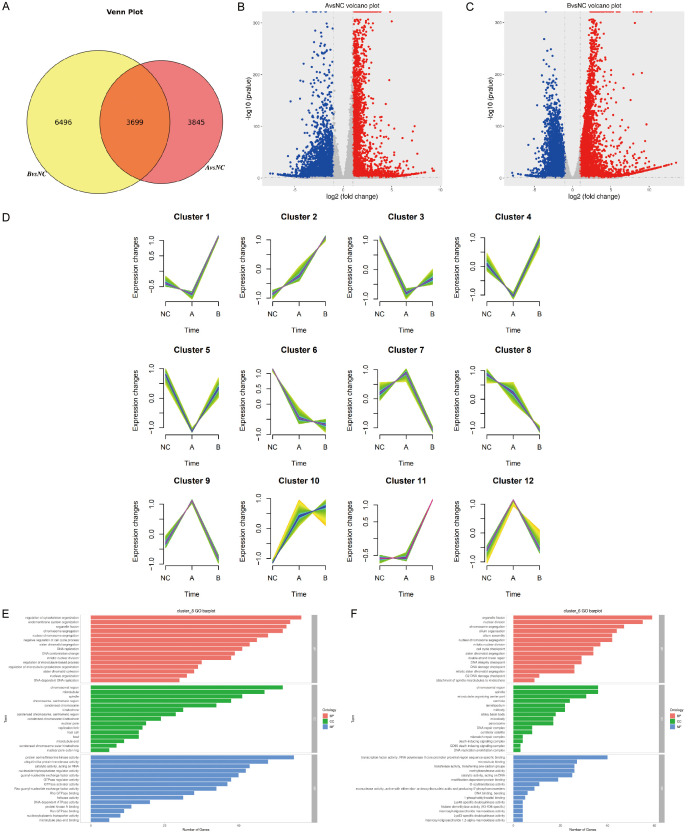
Three stages (no stimulation and stimulation for 12 and 24 h) for transcriptome sequencing were selected to understand the underlying molecular differences induced by Fasudil. In general, the Venn plot showed more differentially expressed genes (DEGs) in the B (24-h) group than in the A (12-h) group (Figure 3A). Interestingly, 3426 upregulated and 4118 downregulated genes were identified in the NC group vs A (12-h) group (Figure 3B), while 5521 upregulated and 4674 downregulated genes were identified in the NC group vs B (24-h) group (Figure 3C). In addition, the total DEGs were clustered into 12 profiles based on the time series, of which 2 profiles, including profiles 6 and 8, displayed a continuous declining trend (Figure 3D). Further GO analysis for profiles 6 and 8 displayed the top 15 categorized biological processes, molecular functions, and cellular compartments. In terms of the biological processes, the tumor proliferation process was the major affected event, including the regulation of cell cycle, DNA replication, and chromosome segregation. In terms of molecular functions, most DEGs were involved in protein serine/threonine kinase activity, ubiquitin-like protein transferase activity, catalytic activity acting on RNA, and transcription factor activity. Additionally, most DEGs were divided into chromosomal region, microtubule, and spindle by cellular compartments (Figure 3E, 3F). On the contrary, the genes having the opposite increasing pattern, such as clusters 10 and 11, were mostly enriched in the metabolic and chromatin process.
Figure 3.
(A) Venn plot showed more differentially expressed genes (DEGs) in the B (24-h) group than in the A (12-h) group. (B) 3426 upregulated and 4118 downregulated genes were identified in the NC group vs A (12-h) group. (C) 5521 upregulated and 4674 downregulated genes were identified in the NC group vs B (24-h) group. (D) Time-series analysis divided all DEGs into 12 clusters. (E, F) GO analysis for cluster 6 (E) and cluster 8 (F) displayed the top 15 categorized biological processes, molecular functions, and cellular compartments. Tumor proliferation process, including the regulation of cell cycle, DNA replication, and chromosome segregation, was the most affected biological event. In terms of molecular functions, most DEGs were involved in protein serine/threonine kinase activity, ubiquitin-like protein transferase activity, catalytic activity acting on RNA, and transcription factor activity. Additionally, most DEGs were divided into chromosomal region, microtubule, and spindle by cellular compartments.
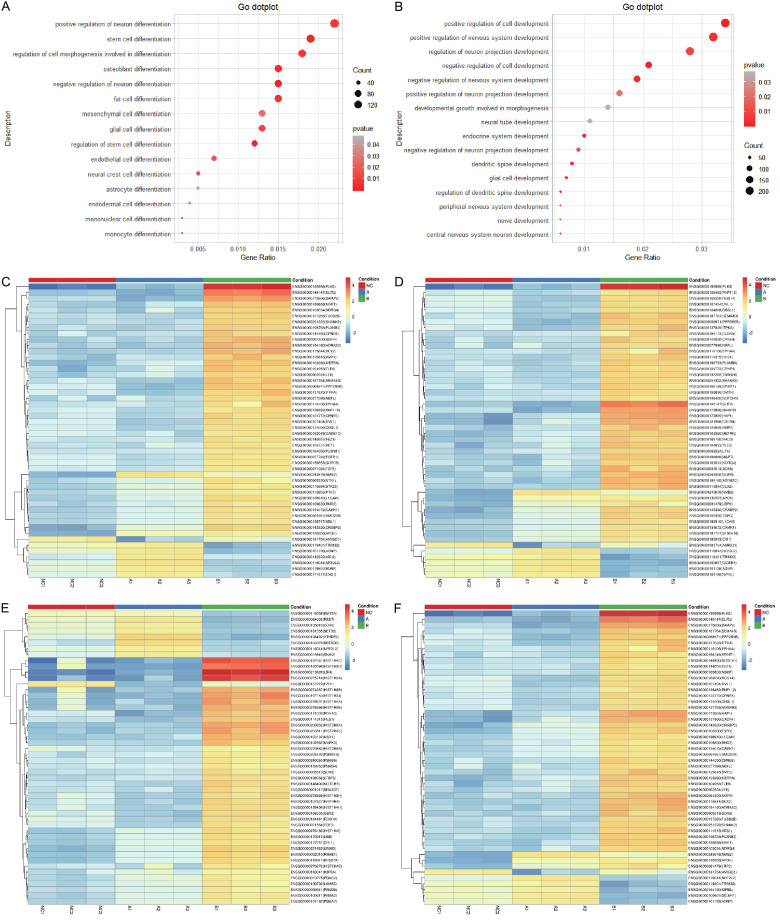
The essence of cell differentiation is selective expression of genes. The biological processes related to cell differentiation and development were further analyzed to investigate whether SCLC cells tended to differentiate after Fasudil stimulation. At least 100 DEGs were involved in the positive regulation of neuron differentiation, stem cell differentiation, and regulation of cell morphogenesis involved in differentiation (Figure 4A), while more than 200 DEGs were involved in the positive regulation of cell development and nervous system development (Figure 4B). The heatmaps representing the differential expression of the top 50 DEGs in the positive regulation of neuron differentiation (Figure 4C), stem cell differentiation (Figure 4D), cell development (Figure 4E), and nervous system development (Figure 4F) were constructed. Of these, Plk5 and Slit2 were significantly enriched and involved in multiple biological processes related to cell differentiation and development. These results indicated that SCLC cells became more mature after Fasudil stimulation.
Figure 4.
(A) At least 100 DEGs were involved in the positive regulation of neuron differentiation, stem cell differentiation, and regulation of cell morphogenesis involved in differentiation. (B) More than 200 DEGs were involved in the positive regulation of cell development and nervous system development. (C-F) The heatmaps representing the differential expression of the top 50 DEGs in the positive regulation of neuron differentiation (C), stem cell differentiation (D), cell development (E), and nervous system development (F) were constructed. Of them, Plk5 and Slit2 were significantly enriched and involved in multiple biological processes related to cell differentiation and development.
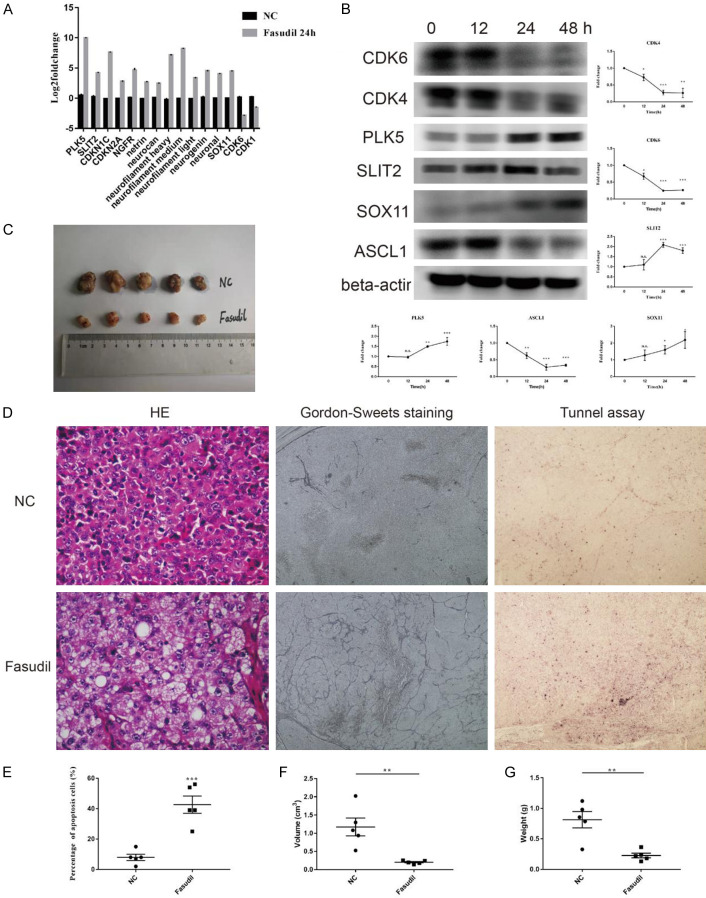
Additionally, the mRNA expression of 15 DEGs was examined by qRT-PCR to validate whether the DEGs in RNA-seq truly reflected the actual transcription level and resulted in protein expression changes. The upregulated genes included PLK5, SLIT2, CDKN1C, CDKN2A, NGFR, netrin 1, neurocan, neurofilament heavy, neurofilament medium, neurofilament light, neurogenin 2, neuronal Per-Arnt-Sim (PAS) domain protein 1, and SOX11, while CDK6 and CDK1 were downregulated (Figure 5A). At the protein level, the expression of CDK4 and CDK6 for cell cycle; ASCL1 for tumor malignancy; and SOX11, PLK5, and SLIT2 for tumor maturation was investigated. The findings revealed that the expression levels of ASCL1, CDK4, and CDK6 decreased, while those of SOX11, PLK5, and SLIT2 increased after Fasudil stimulation (Figure 5B). The protein expression patterns of CDK6, SOX11, PLK5, and SLIT2 were consistent with the trend changes of DEGs in RNA-seq and qRT-PCR.
Figure 5.
(A) Examination of DEGs in RNA-seq by qRT-PCR. The upregulated genes included PLK5, SLIT2, CDKN1C, CDKN2A, NGFR, netrin 1, neurocan, neurofilament heavy, neurofilament medium, neurofilament light, neurogenin 2, neuronal Per-Arnt-Sim (PAS) domain protein 1, and SOX11, while CDK6 and CDK1 were downregulated (n = 3). (B) Western blot detected that the levels of ASCL1, CDK4, and CDK6 were decreased while those of SOX11, PLK5, and SLIT2 were increased after Fasudil stimulation (n = 3). (C) Representative gross image showed Fasudil inhibited xenograft SCLC tumor growth (n = 5). (D-G) HE staining showed tumors in the Fasudil group had more abundant interstitial substances and loose cell arrangement. Gordon-Sweet staining showed more reticular fibers in the Fasudil group (D). TUNEL assay showed that Fasudil could promote tumor apoptosis (D, E), with a significant reduction in tumor volume (F) and weight (G) (magnification × 200). Data were presented as mean ± SEM; *P < 0.05, **P < 0.01, and ***P < 0.001.
Therapeutic effect of Fasudil hydrochloride in vivo
In vivo, Fasudil inhibited xenograft SCLC tumor growth (Figure 5C). Tumors in the Fasudil group had more abundant interstitial substances and loose cell arrangement (Figure 5D). Gordon-Sweet staining showed more reticular fibers in the Fasudil group, indicating higher structural maturity. Moreover, the TUNEL assay demonstrated that Fasudil could promote tumor apoptosis in vivo (Figure 5D, 5E), accompanied by a significant reduction in tumor volume (Figure 5F) and weight (Figure 5G), compared with that in controls.
Discussion
SCLC is one of the deadliest cancers nowadays. Therapeutic options for SCLC develop extremely slowly, which has made the treatment of SCLC quite challenging for decades. Induction-differentiation therapy has been widely and successfully applied in various hematological neoplasms. However, few inducers were reported in lung cancer, especially in SCLC. This study reported that Fasudil hydrochloride, a potent and selective ROCK1/2 inhibitor, might be a potential inducer for SCLC differentiation therapy.
The expression and role of ROCK1/2 have been broadly investigated in many tumors, especially in non-small-cell lung cancer (NSCLC). ROCK1 and ROCK2 are required for NSCLC anchorage-independent growth and invasion [19,20]. This study investigated the expression of ROCK1/2 in lung neuroendocrine tumors, including TC, AC, LCNEC, and SCLC. Of these, SCLC was the most poorly differentiated tumor. Interestingly, the results of this study showed that the ROCK1/2 positive rate was distinctly higher in SCLC but almost negative in other lung neuroendocrine tumors. In ASCL1-positive, namely classic SCLC subtype, patients, ROCK1 has a statistically stronger prognostic value, indicating a possible regulation correlation between ROCK1 and ASCL1. ASCL1 is essential for the development and progression of SCLC [2]. ASCL1-silenced SCLC cell line lost tumorigenicity in nude mice [21]. Later, it was proved in the present study that ROCK1 inhibition by Fasudil resulted in the downregulation of the expression of ASCL1, implying that Fasudil treatment might reduce SCLC malignancy and aggressiveness.
The cell cycle analysis showed that Fasudil induced the reduction of cells in the S phase and accumulation of cells in both the G0/G1 phase and G2/M phase. In RNA-seq and GO analysis, DEGs were significantly enriched in the cell cycle process and G2-M DNA damage checkpoint process after Fasudil stimulation, both of which showed a declining trend. Besides, the protein expression of CDK4, CDK6, cyclin D1, and c-myc was also downregulated. Generally, CDK4, CDK6, and cyclin D1 are involved in the G1/S transition [22]. Moreover, c-myc regulates the CDK1/cyclin B1-dependent G2/M cell cycle progression by histone H4 acetylation [23]. Compiling the aforementioned results, Fasudil could distinctly induce cell cycle arrest in both the G1/S and G2/M phases. In addition, cell cycle arrest was rapidly followed by apoptosis. In this study, apoptosis of Fasudil-induced SCLC cells showed dependence on the alimentary deficiency. When the culture medium was exchanged, the apoptotic effect was blocked or reversed by the relief of starvation. Tumors were more tolerant to alimentary deficiency compared with normal tissues. This phenomenon suggested tumor maturation after Fasudil stimulation.
On the contrary, RNA-seq has identified hundreds of DEGs involved in the positive regulation of neuron differentiation, stem cell differentiation, cell development, and nervous system development after Fasudil stimulation. Among the upregulated genes, Plk5 and Slit2 were the most obviously enriched and upregulated genes. Further, PLK5 and SLIT2 increased at the protein level too. PLK5 is an antiproliferative PLK family member specifically expressed in the eye and the brain. It is involved in neuron differentiation and is required for axonal growth in neuroblasts. It is expressed in differentiated cells, such as neurons and glia, but is downregulated in human brain tumors by promoter hypermethylation [24-26]. SLIT2 is classified as a major tumor suppressor gene in different cancer types [27-29]. Otherwise, Slit2 is a b-catenin/Ctnnb1-dependent retrograde signal for presynaptic differentiation [30], while it also regulates osteoblast [31] and enteroendocrine cell differentiation [32]. Furthermore, RNA-seq showed that several genes in the SOX family were upregulated after Fasudil stimulation, including SOX11 (data not shown, but was available in the supplement file of DEGs). SOX11 plays a crucial role when neural progenitor cells establish a neuronal phenotype. It is identified in neural committed cells rather than non-committed precursor cells [33]. Thus collectively, the transcription spectrum changes, including upregulation of neuron differentiation and development-related genes, such as PLK5, SLIT2, and SOX11, after Fasudil stimulation indicated tumor cell differentiation, maturation, and phenotype transition.
Finally, the in vivo experiment of Fasudil showed efficacy in reducing tumor growth. In morphology, more abundant interstitial substances and loose cell arrangements were seen in the Fasudil-treated group. The tumor nests surrounded by reticular fibers were a morphological feature of TC rather than SCLC [34]. Additionally, Gordon-Sweet reticular fiber staining showed more reticular septums in the Fasudil-treated group, demonstrating that Fasudil could promote SCLC structural maturation in vivo. The TUNEL assay further confirmed that Fasudil could induce apoptosis in vivo.
Collectively, this study demonstrated high expression of ROCK1/2 in SCLC, and ROCK1/2 could be potential therapeutic targets in SCLC. Fasudil, the ROCK1/2 inhibitor routinely used in cardiocerebrovascular diseases, might have a novel application in SCLC differentiation therapy.
Acknowledgements
Supported by grants 81570254, 81770263, 81974016 and 31271040 from the National Natural Science Foundation of China.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Gazdar AF, Bunn PA, Minna JD. Small-cell lung cancer: what we know, what we need to know and the path forward. Nat Rev Cancer. 2017;17:725–737. doi: 10.1038/nrc.2017.87. [DOI] [PubMed] [Google Scholar]
- 2.Rudin C, Poirier J, Byers L, Dive C, Dowlati A, George J, Heymach J, Johnson E, Lehman J, MacPherson D, Massion P, Minna J, Oliver T, Quaranta V, Sage J, Thomas R, Vakoc C. Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data. Nat Rev Cancer. 2019;19:289–297. doi: 10.1038/s41568-019-0133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horn L, Mansfield A, Szczęsna A, Havel L, Krzakowski M, Hochmair M, Huemer F, Losonczy G, Johnson M, Nishio M, Reck M, Mok T, Lam S, Shames D, Liu J, Ding B, Lopez-Chavez A, Kabbinavar F, Lin W, Liu S. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379:2220–2229. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 4.Imai H, Yamada Y, Minemura H, Sugiyama T, Kotake M, Kaira K, Kanazawa K, Nakamura Y, Kasai T, Shibata Y, Kaburagi T, Minato K. Topotecan monotherapy for the treatment of relapsed small cell lung cancer in elderly patients: a retrospective analysis. Thorac Cancer. 2018;9:1699–1706. doi: 10.1111/1759-7714.12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hellmann M, Callahan M, Awad M, Calvo E, Ascierto P, Atmaca A, Rizvi N, Hirsch F, Selvaggi G, Szustakowski J, Sasson A, Golhar R, Vitazka P, Chang H, Geese W, Antonia S. Tumor mutational burden and efficacy of nivolumab monotherapy and in combination with ipilimumab in small-cell lung cancer. Cancer Cell. 2018;33:853–861. e4. doi: 10.1016/j.ccell.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schofield A, Bernard O. Rho-associated coiled-coil kinase (ROCK) signaling and disease. Crit Rev Biochem Mol. 2013;48:301–316. doi: 10.3109/10409238.2013.786671. [DOI] [PubMed] [Google Scholar]
- 7.Watzlawick R, Sena E, Dirnagl U, Brommer B, Kopp M, Macleod M, Howells D, Schwab J. Effect and reporting bias of RhoA/ROCK-blockade intervention on locomotor recovery after spinal cord injury. JAMA Neurol. 2014;71:91–99. doi: 10.1001/jamaneurol.2013.4684. [DOI] [PubMed] [Google Scholar]
- 8.Sanz-Moreno V, Gaggioli C, Yeo M, Albrengues J, Wallberg F, Viros A, Hooper S, Mitter R, Feral CC, Cook M, Larkin J, Marais R, Meneguzzi G, Sahai E, Marshall CJ. ROCK and JAK1 signaling cooperate to control actomyosin contractility in tumor cells and stroma. Cancer Cell. 2011;20:229–245. doi: 10.1016/j.ccr.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 9.Shingo T, Hironori K, Manabu N. Eph/ephrin reverse signalling induces axonal retraction through RhoA/ROCK pathway. J Biochem. 2015;158:245–252. doi: 10.1093/jb/mvv042. [DOI] [PubMed] [Google Scholar]
- 10.Kassianidou E, Hughes J, Kumar S. Activation of ROCK and MLCK tunes regional stress fiber formation and mechanics via preferential myosin light chain phosphorylation. Mol Biol Cell. 2017;28:3832–3843. doi: 10.1091/mbc.E17-06-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li S, Tuft B, Xu L, Polacco M, Clarke J, Guymon C, Hansen M. Microtopographical features generated by photopolymerization recruit RhoA/ROCK through TRPV1 to direct cell and neurite growth. Biomaterials. 2015;53:95–106. doi: 10.1016/j.biomaterials.2015.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minase T, Ishima T, Itoh K, Hashimoto K. Potentiation of nerve growth factor-induced neurite outgrowth by the ROCK inhibitor Y-27632: a possible role of IP3 receptors. Eur J Pharmacol. 2010;648:67–73. doi: 10.1016/j.ejphar.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Chan C, Roberts C, Steeves J, Tetzlaff W. Aggrecan components differentially modulate nerve growth factor-responsive and neurotrophin-3-responsive dorsal root ganglion neurite growth. J Neurosci Res. 2008;86:581–592. doi: 10.1002/jnr.21522. [DOI] [PubMed] [Google Scholar]
- 14.Shaw P, Sang A, Wang Y, Ho D, Douglas C, Dia L, Goldberg J. Topical administration of a Rock/Net inhibitor promotes retinal ganglion cell survival and axon regeneration after optic nerve injury. Exp Eye Res. 2016;158:33–42. doi: 10.1016/j.exer.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohbuchi M, Kimura T, Nishikawa T, Horiguchi T, Fukuda M, Masaki Y. Neuroprotective effects of fasudil, a rho-kinase inhibitor, after spinal cord ischemia and reperfusion in rats. Anesth Analg. 2018;126:815–823. doi: 10.1213/ANE.0000000000002602. [DOI] [PubMed] [Google Scholar]
- 16.Kramer B, Tropitzsch A, Müller M, Löwenheim H. Myelin-induced inhibition in a spiral ganglion organ culture - approaching a natural environment in vitro. Neuroscience. 2017;357:75–83. doi: 10.1016/j.neuroscience.2017.05.053. [DOI] [PubMed] [Google Scholar]
- 17.Paintlia A, Paintlia M, Singh A, Singh I. Modulation of Rho-ROCK signaling pathway protects oligodendrocytes against cytokine toxicity via PPAR-α-dependent mechanism. Glia. 2013;61:1500–1517. doi: 10.1002/glia.22537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi N, Nobusue H, Shimizu T, Sugihara E, Yamaguchi-Iwai S, Onishi N, Kunitomi H, Kuroda T, Saya H. ROCK inhibition induces terminal adipocyte differentiation and suppresses tumorigenesis in chemoresistant osteosarcoma cells. Cancer Res. 2019;79:3088–3099. doi: 10.1158/0008-5472.CAN-18-2693. [DOI] [PubMed] [Google Scholar]
- 19.Kang C, Han H, Lee H, Kim S, Lee E. Rho-associated kinase signaling is required for osteopontin-induced cell invasion through inactivating cofilin in human non-small cell lung cancer cell lines. Bioorg Med Chem Lett. 2015;25:1956–1960. doi: 10.1016/j.bmcl.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 20.Kang C, Lee H, Kim S, Lee E. Zerumbone suppresses osteopontin-induced cell invasion through inhibiting the FAK/AKT/ROCK pathway in human non-small cell lung cancer A549 cells. J Nat Prod. 2015;79:156–160. doi: 10.1021/acs.jnatprod.5b00796. [DOI] [PubMed] [Google Scholar]
- 21.Borromeo M, Savage T, Kollipara R, He M, Augustyn A, Osborne JK, Girard L, Minna J, Gazdar A, Cobb M, Johnson E. ASCL1 and NEUROD1 reveal heterogeneity in pulmonary neuroendocrine tumors and regulate distinct genetic programs. Cell Rep. 2016;16:1259–1272. doi: 10.1016/j.celrep.2016.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sancar A, Lindsey-Boltz LA, Ünsal-Kaçmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- 23.Yang Y, Xue K, Li Z, Zheng W, Dong W, Song J, Sun S, Ma T, Li W. c-Myc regulates the CDK1/cyclin B1 dependent-G2/M cell cycle progression by histone H4 acetylation in Raji cells. Int J Mol Med. 2018;41:3366–3378. doi: 10.3892/ijmm.2018.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andrysik Z, Bernstein W, Deng L, Myer D, Li Y, Tischfield J, Stambrook P, Bahassi EM. The novel mouse Polo-like kinase 5 responds to DNA damage and localizes in the nucleolus. Nucleic Acids Res. 2010;38:2931–2943. doi: 10.1093/nar/gkq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Cárcer G, Escobar B, Higuero A, García L, Ansón A, Pérez G, Mollejo M, Manning G, Meléndez B, Abad-Rodríguez J, Malumbres M. Plk5, a polo box domain-only protein with specific roles in neuron differentiation and glioblastoma suppression. Mol Cell Biol. 2011;31:1225–1239. doi: 10.1128/MCB.00607-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Cárcer G, Manning G, Malumbres M. From Plk1 to Plk5: functional evolution of polo-like kinases. Cell Cycle. 2011;10:2255–2262. doi: 10.4161/cc.10.14.16494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dallol A, Morton D, Maher E, Latif F. SLIT2 axon guidance molecule is frequently inactivated in colorectal cancer and suppresses growth of colorectal carcinoma cells1. Cancer Res. 2003;63:1054–1058. [PubMed] [Google Scholar]
- 28.Shi R, Yang Z, Liu W, Liu B, Xu Z, Zhang Z. Knockdown of Slit2 promotes growth and motility in gastric cancer cells via activation of AKT/β-catenin. Oncol Rep. 2013;31:812–818. doi: 10.3892/or.2013.2887. [DOI] [PubMed] [Google Scholar]
- 29.Yu J, Zhang X, Kuzontkoski P, Jiang S, Zhu W, Li D, Groopman J. Slit2N and Robo4 regulate lymphangiogenesis through the VEGF-C/VEGFR-3 pathway. Cell Commun Signal. 2014;12:25. doi: 10.1186/1478-811X-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu H, Barik A, Lu Y, Shen C, Bowman A, Li L, Sathyamurthy A, Lin TW, Xiong WC, Mei L. Slit2 as a β-catenin/Ctnnb1-dependent retrograde signal for presynaptic differentiation. Elife. 2015;4:e7266. doi: 10.7554/eLife.07266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun H, Dai K, Tang T, Zhang X. Regulation of osteoblast differentiation by slit2 in osteoblastic cells. Cells Tissues Organs. 2008;190:69–80. doi: 10.1159/000178020. [DOI] [PubMed] [Google Scholar]
- 32.Nagy P, Szatmari Z, Sándor G, Lippai M, Hegedűs K, Juhasz G. Drosophila Atg16 promotes enteroendocrine cell differentiation via regulation of intestinal Slit/Robo signaling. Development. 2017;144:147033. doi: 10.1242/dev.147033. [DOI] [PubMed] [Google Scholar]
- 33.Bergsland M, Werme M, Malewicz M, Perlmann T, Muhr J. The establishment of neuronal properties is controlled by Sox4 and Sox11. Gene Dev. 2007;20:3475–3486. doi: 10.1101/gad.403406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caplin M, Baudin E, Ferolla P, Filosso P, Garcia-Yuste M, Lim E, Oberg K. Pulmonary neuroendocrine (carcinoid) tumors: European neuroendocrine tumor society expert consensus and recommendations for best practice for typical and atypical pulmonary carcinoid. Ann Oncol. 2015;26:1604–1620. doi: 10.1093/annonc/mdv041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.