FIG 1.
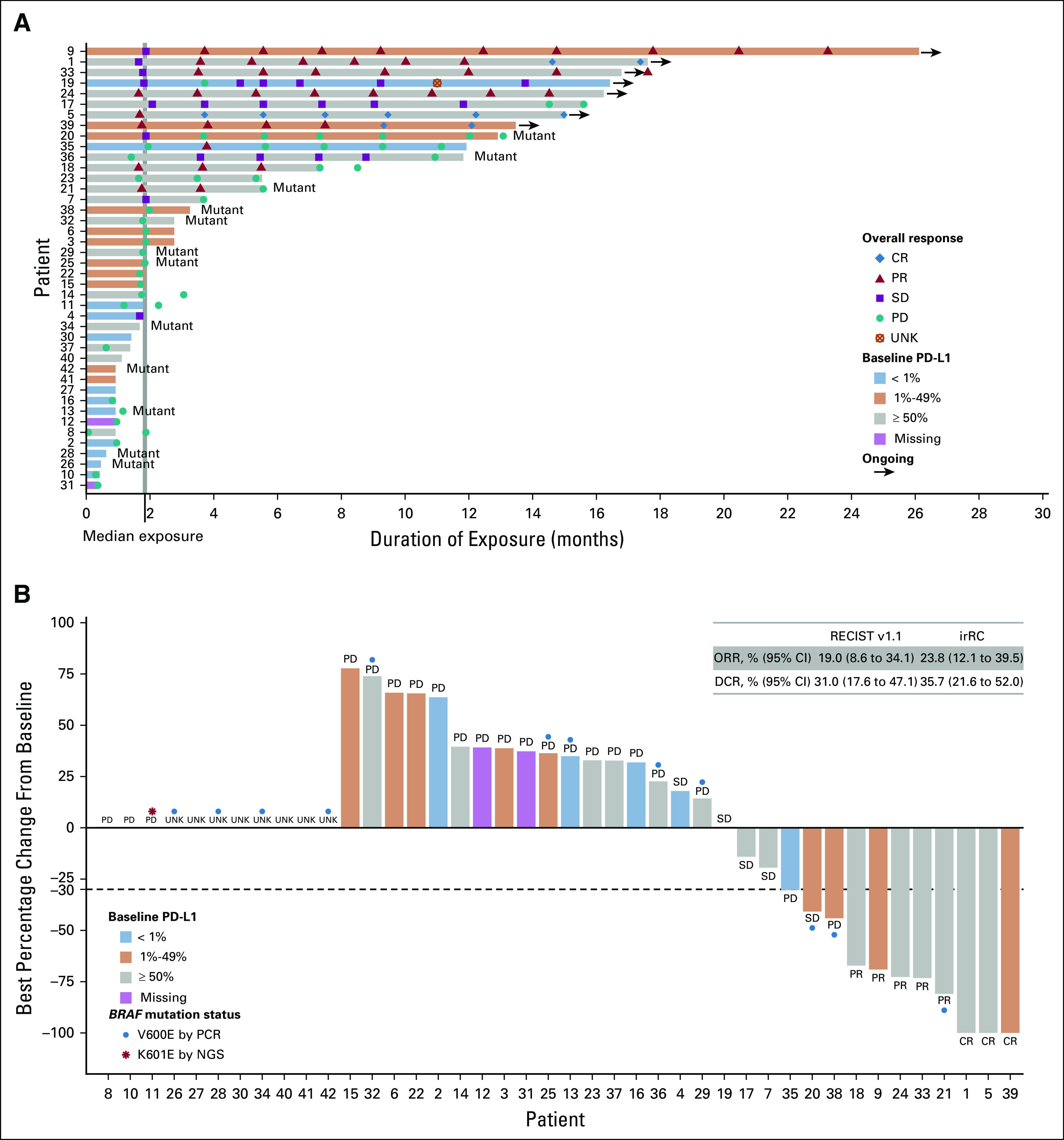
Duration of exposure to spartalizumab and percentage change from baseline in sum of diameters of target lesions, by programmed death-ligand 1 (PD-L1) expression at baseline. (A) Duration of exposure to spartalizumab. Mutant denotes BRAF mutation. (B) Best percentage change from baseline in sum of diameters of target lesions. Thirty-one patients were evaluable for best percentage change; 11 patients were not evaluable because of discontinuation or death before first postbaseline assessment (n = 8) or missing postbaseline assessment (n = 3). Best overall response by RECIST v1.1 is indicated. Where available, BRAF mutation status is indicated. Patient number refers to number in Data Supplement. CR, complete response; DCR, disease control rate [CR + PR + SD]; irRC, immune-related response criteria; NGS, next-generation sequencing; ORR, overall response rate [CR + PR]; PCR, polymerase chain reaction; PD, progressive disease; PR, partial response; SD, stable disease; UNK, unknown.
