Abstract
Venous thromboembolism (VTE) is a major health problem in patients with cancer. Cancer augments thrombosis and causes cancer-associated thrombosis (CAT) and vice versa thrombosis amplifies cancer progression, termed thrombosis-associated cancer (TAC). Risk factors that lead to CAT and TAC include cancer type, chemotherapy, radiotherapy, hormonal therapy, anti-angiogenesis therapy, surgery, or supportive therapy with hematopoietic growth factors. There are some other factors that have an effect on CAT and TAC such as tissue factor, neutrophil extracellular traps (NETs) released in response to cancer, cancer procoagulant, and cytokines. Oncogenes, estrogen hormone, and thyroid hormone with its integrin αvβ3 receptor promote angiogenesis. Lastly, patient-related factors can play a role in development of thrombosis in cancer. Low-molecular-weight heparin and direct oral anticoagulants (DOACs) are used in VTE prophylaxis and treatment rather than vitamin K antagonist. Now, there are new directions for potential management of VTE in patients with cancer such as euthyroid, blockade of thyroid hormone receptor on integrin αvβ3, sulfated non-anticoagulant heparin, inhibition of NETs and stratifying low and high-risk patients with significant bleeding problems with DOACs.
Keywords: angiogenesis, anticoagulants, cancer, coagulation, heparins, inflammation, integrin αvβ3, non-anticoagulant heparin, oral anticoagulant, thrombosis, thyroid hormone
Introduction
In 1865, a French physician named Armand Trousseau described the first association between thrombosis and cancer, which was later called the Trousseau syndrome. Several clinical trials have revealed the link between venous thrombosis and cancer and demonstrated an increase in the prevalence of venous thrombosis in patients with cancer. In 1977, Sack et al. discovered that the Trousseau syndrome was chronically disseminated vascular coagulation associated with non-bacterial thrombotic endocarditis and arterial thrombosis in malignant patients.1 A vicious cycle between cancer and thrombosis is termed cancer-associated thrombosis (CAT) where cancer amplifies the risk of thrombosis. Vice versa, where thrombosis amplifies the cancer progression, this is termed thrombosis-associated cancer (TAC) (Figure 1). Venous thromboembolism (VTE) includes the conditions of deep vein thrombosis (DVT) and pulmonary embolism.2 The most important VTE-related risk is cancer; 20%-30% of VTE occurrences are assumed to be associated with cancer.3 The risk of developing arterial and venous thromboembolism is significantly increased in patients with cancer.4 Mortality is increased in cancer patients with VTE compared to cancer patients without VTE.5 It has been reported that the annual incidence of VTE in patients with cancer is 0.5% compared to 0.1% in the non-cancer population.6 Survival of patients with VTE was poorer in CAT.7
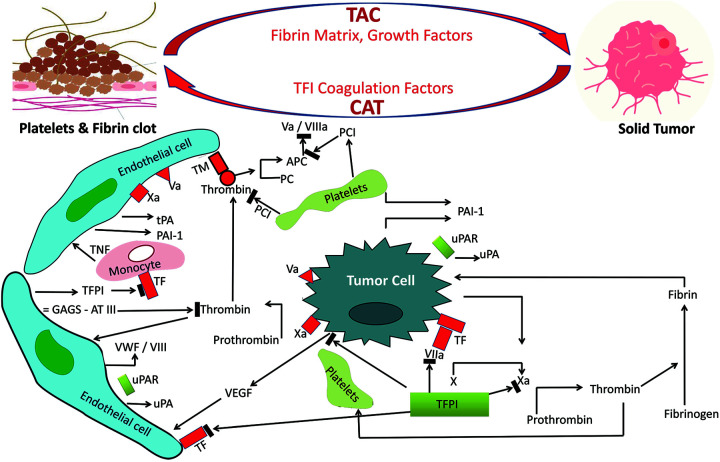
Figure 1.
Representation of the reciprocal links between cancer and thrombosis. Tumor cells can express procoagulant factors, such as tissue factor (TF), that trigger the cascade of coagulation, which would be suppressed by mechanisms that induce endothelial tissue factor pathway inhibitors (TFPI). Tumor cells interact with endothelial cells, platelets, and leukocytes and these interactions induce inflammatory cytokines and pro-angiogenic factors such as vascular endothelial growth factor (VEGF). Neutrophil attraction contributes to the accumulation of neutrophil extracellular traps (NETs) and triggers adhesion, activation, and generation of thrombin. In general, the adherence of cancer to the endothelium causes the release of procoagulants and formation of thrombus, leading to CAT occurrence. TF also triggers the generation of factor VIIa, factor Xa, and thrombin (IIa), which activate platelets and induce protease-activated receptor-mediated signaling. The plasminogen activator (PA) system is expressed on platelets and it consists of plasminogen activators: urokinase plasminogen activator (uPA), tissue plasminogen activator (tPA), cell membrane receptor for uPA (uPAR), and the plasminogen activator inhibitors plasminogen activator inhibitor 1 (PAI-1) and plasmin. The PA system has an effect on cell adhesion and migration, which is particularly important in cancer progression. Also, TF suppress vasculoprotective molecules, such as thrombomodulin (TM). Release of von willebrand factor (VWF) promotes recruitment, adhesion, and activation of platelets and leukocytes with formation of NETs. abbreviations: APC, activated protein C; GAGS, glycosaminoglycans heparin sulfate; PC, protein C; PCI, protein C inhibitor; TNF, tumor necrosis factor-alpha.
This review focuses on the interplay between cancer and thrombosis and the impact on a cancer patient’s management, with special emphasis on treatment of CAT.
Risk Factors for CAT
Cancer Type and Stage
Cancer is a heterogeneous illness because it has different types and stages, which may have effects on VTE.8 CAT is common in many types of cancer such as pancreatic, brain, lung, and ovarian cancers.9–11 Patients with prostate cancer usually have a relatively low risk,12 and patients with breast cancer are reported to develop VTE 3 to 4 times more often than patients of equivalent age without breast cancer.9 The aggressiveness of cancer, early metastatic development, and short survival were particularly associated with a high incidence of VTE.13
The risk of VTE occurrence is higher in advanced metastatic cancer than in localized cancer.14 Previous studies confirmed that tumor grade and stage were significantly associated with risk of venous thrombosis.9 Moreover, the risk of VTE was high in the first 3 months after the cancer diagnosis and then decreased gradually during the 10 years after the cancer diagnosis.11 This may be because multiple cancer therapies (e.g., chemotherapy, radiotherapy) boost VTE risk. Also, a percentage of patients with cancer enter a remission phase, which reduces the danger of VTE. Another reason is that a large proportion of cancer patients will succumb to the disease over time. This contesting event (death) prevents the observation of thrombotic events.8
Cancer Treatment
Tumor pressure on blood vessels results in stasis and VTE. Some medications that are used in treatment of cancer are related to high risk of thrombosis. Examples of these medications are hormonal therapy such as tamoxifen, chemotherapy (e.g., paclitaxel, doxorubicin, thalidomide), molecular targeted therapy such as osimertinib, anti-angiogenesis therapy (e.g., bevacizumab, ramucirumab), immunotherapies and radiation therapies.15
Chemotherapy
Using combination therapy of chemotherapeutic drugs increases the risk of arterial thrombosis occurrence in patients with breast cancer, but the mechanism is not clear.16 Also, chemotherapy increases the risk of VTE17 and produces a prothrombotic condition through a number of distinct mechanisms18 that can harm the endothelium directly,19 decrease endogenous anticoagulant substances, and/or boost the procoagulant protein.20 Chemotherapy also can cause apoptosis and cytokine release, which can in turn lead to enhanced endothelial and monocyte tissue factor (TF) expression that is considered as the physiologic initiator of coagulation.21 Chemotherapy can ultimately lead to platelet activation.22
Both cytotoxic and targeted chemotherapy are associated with VTE. For example, cisplatin induces endothelial cell apoptosis and results in the release of procoagulant endothelial microparticles.23 L-asparaginase causes depletion of key proteins in the regulation of the coagulation pathway24—therefore VTE is a complication in patients with acute lymphoblastic leukemia receiving asparaginase inhibitor chemotherapy and these symptomatic VTEs can be reduced by low-molecular-weight heparin (LMWHs).25 5-Fluorouracil causes depletion of protein C, increases thrombin activity,26 and damages endothelial cells to promote thrombus formation.26 Another example is tamoxifen, an anti-estrogen drug. Its estrogenic activity might elevate the risk of VTE through depletion of protein C and protein S.27,28
Not only is chemotherapy associated with increased VTE risk but also cancer type is associated with further increase in VTE risk.29 Patients with advanced pancreatic ductal adenocarcinoma have high risk of VTE, and chemotherapy can further increase that risk. Moreover, thromboprophylaxis with LMWH for 3 months decreased that risk, however, further anticoagulation expansion may be helpful to limit a future thromboembolic risk.30 Because there are growing risks of CAT due to additional new antineoplastic drugs, immunotherapies, or chemotherapy combinations, a prospective registry named TESCO and promoted by the Spanish Society of Medical Oncology data highlight the evolution of CAT, with new agents and thrombotic risk factors.31
Radiotherapy
Radiotherapy can be used when VTE risk is high in early phases of cancer,11 or as part of localized treatment, with or without chemotherapy for localized tumors.32 As a result, there is increasing evidence that radiotherapy could affect the result of VTE anticoagulant therapy in cancer patients.33 Ionizing radiation influences protein C pathway and its thrombomodulin interaction.34 After radiation, procoagulant factors with activated factor VIII, pro-inflammatory nuclear factor kappa B, increased D-dimers and pro-thrombin fragments prompt pro-coagulant response.35 Radiation also induces primary hemostasis through TF and von Willebrand activation36 leading to endothelial dysfunction and thrombosis. Also, an in vitro study has shown that tumor irradiation activates cancer cell integrin αvβ3,37 which has a pivotal role in CAT, and that role will be discussed later in this review.
Hormonal therapy
Use of hormone therapy is a clear risk factor for the development of cancer38 and it elevates VTE risk,39 which can enhance the link between CAT and TAC.
Surgery
Major surgery is a well-known risk factor for VTE, and patients who develop VTE in the post-operative period have a higher mortality rate.40 Additionally, the length of surgery has been associated with an increased risk of VTE.41 A prothrombotic state may occur due to several processes including enhanced levels of fibrinogen, thrombin generation, activation of TF, decreased fibrinolysis and hemostasis.42 Furthermore, patient immobility after an operation can lead to venous stasis and cancer procoagulants’ overexpression that causes immediate damage to the vascular endothelium.19 Other factors that may increase the risks from surgery on VTE formation are pelvic dissection, the patient’s position during surgery, cancer, advanced age, heart or respiratory failure.43
Supportive therapy with hematopoietic growth factors
Previous studies showed that patients treated with erythropoiesis-stimulant agents in addition to red blood cell transfusions have a higher risk of VTE than do patients without additional erythropoiesis-stimulant agents.44,45 Erythropoietin is found to be an activator to platelets and enhances factor VIII and thrombin generation and decreases protein C and protein S.46 Erythropoietin also promotes signaling pathways in endothelial cells and enhances their thrombogenicity.47
Hormonal Risk Factors
Thyroid hormones
Thyroid hormones are key regulators of essential cellular processes including proliferation, differentiation, apoptosis, and metabolism.48 A prospective study reported that subclinical hyperthyroidism was associated with enhanced risk of cancer, particularly in lung, prostate, ovarian, pancreatic, and renal carcinoma.49
Thyroid hormone has an effect on cancer cell proliferation and angiogenesis.50,51 The intracellular metabolically active hormone in normal cells is 3,5,3′-triiodo-L-thyronine (T3) from prohormone L-thyroxine (T4). The thyroid hormone action is done by genomic and non-genomic actions. Genomic action is initiated when T3 binds to thyroid receptors that interact with specific responding elements. Non-genomic actions modulate gene transcription by activating pathways and other transcription factors.52,53 The availability of T4 is the pivotal step for activation of the integrin and consequent trans-membrane signaling into the cell. T3 and T4 bind to the plasma membrane protein integrin αvβ3, which mediates the signal across the membrane. At physiological concentrations, T4 is the primary ligand at αvβ3.54
Thyroid hormone also has a pro-angiogenic effect initiated by several thyroid hormone analogs affecting coagulation by acting on platelet integrin αvβ3.55 Thyroid hormone stimulates the aggregation of platelet and the release of platelets’ adenosine triphosphate (ATP).56 All of the above may explain the association between thyroid hormone, platelets, and CAT.57 Concepts highlighted for future directions with regard to the role of thyroid hormone and integrin αvβ in CAT warrant clinical investigations.
Estrogen hormone
Estrogen has been associated with an increased risk of thromboembolic events in patients using combined hormone replacement or estrogens.38,58 Venous thromboembolism risk increases in patients with cancers such as breast cancer59 and endometrial cancer, which are positively correlated with serum estrogen levels.60 Estrogens have pleiotropic effects due to a large tissue distribution of their receptors61 in many tissues such as platelets with different effects.62 A high level of estrogen enhances the secretion of angiogenic cytokines such as VEGF that influence the expression of integrin αvβ3, which in turn contributes to angiogenesis. By influencing angiogenesis, these angiogenic factors affect the growth of cancer such as breast and ovarian.63,64
Molecular Risk Factors
Oncogenes
CAT may be activated directly or indirectly through an oncogenic process.65 The indirect effect of oncogenes may happen through vascular invasion, metastasis, bleeding, vascular permeability, angiogenesis,65 recruitment of inflammatory cells66 and extracellular vesicles (EVs)67 that may reprogram coagulant phenotypes of endothelial cells68 or leukocytes.67 The direct effect of oncogenes on CAT includes the negative effect of an epidermal growth factor receptor (EGFR) antagonist on the expression of TF in cancer cells.65
Examples for these oncogenes are K-RAS oncogene and p53 tumor suppressor gene.69 A previous study reported a link between integrin αvβ3 and p53 activity in that blockade of αvβ3 suppresses the expression and/or activity of p53.70 K-RAS alters the levels of crucial angiogenic mediators such as VEGF or thrombospondin71 and affects multiple facets of the cancer,72 recruitment of inflammatory cells,66 immune responses,73 and CAT.74 Other oncogenes may regulate TF, such as MET proto-oncogene EGFR, and erb-b2 receptor tyrosine kinase 2 (ERBB2).75
Mechanisms Involved in CAT
Platelets
Cancer cells can activate platelets by direct and indirect mechanisms that lead to CAT.76,77 Tumor-cell induced platelet aggregation (TCIPA) was also linked to greater metastatic potential.76 A significant TCIPA mechanism is secretion of thrombin by cancer cell procoagulants that turn fibrinogen into fibrin. Another mechanism is the activation of coagulation factors V, VIII, XI, XIII, and protease-activated receptors (PAR receptors) that are highly expressed on platelets.78 Tumor cells also express adenosine diphosphate (ADP) that activates platelets to release more ADP and thus activates more platelets.79 Platelets also express TF on their membrane, contributing to CAT.80 Platelets have many growth factors81 that are also present in the tumor microenvironment82 and cause development of the tumor and support angiogenesis.83
Coagulation
Together with cells and platelets, TF is important for normal hemostasis. Coagulation occurs in 3 overlapping phases: initiation, amplification, and propagation.84 In the initiation phase, TF binds to factor VIIa to activate factor IX and factor X. Activation of factor IX works as the link between the extrinsic and intrinsic pathways. Factor Xa then binds to factor II to form thrombin. The amplification phase starts because the amount of thrombin generated is not enough to form a widespread clot, therefore positive feedback loops are present to bind thrombin with platelets. In the propagation phase, enzyme complexes (tenase complex and prothrombinase complex) on the platelet surface support high amounts of thrombin generation and platelet activation.85 This occurs to ensure continuous generation of thrombin and subsequently fibrin generation and polymerization to form a stable clot.86
Tissue factor
The main cause of coagulation in patients with cancer is TF expression on the surface of cancer cells87 as shown in Figure 2. The coagulation extrinsic pathway is initiated by TF because it binds to factor VII and promotes proteolysis and activation to factor VIIa.88 The binary TF/factor VIIa complex activates factor IX and factor X, leading to the development of thrombin, culminating in the production of fibrin.89 The existence of TF in the blood as a component of EVs from vascular cells, and subsequently from tumor cells, increases the prothrombotic status of patients with cancer.90 Other cancer-related phenomena, such as the epithelial-mesenchymal transition, may also trigger the release of EVs containing TF.68
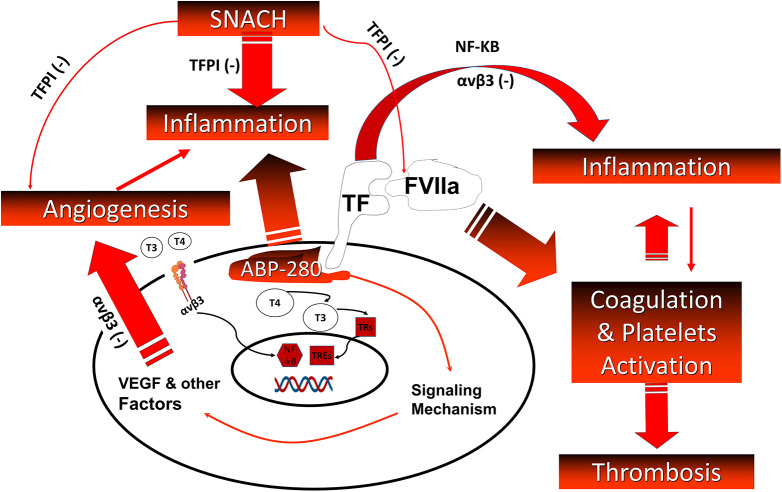
Figure 2.
Relationship between integrin αvβ3, thyroid hormones, TF/FVIIa, angiogenesis, inflammation, and thrombus formation. When TF is released, it activates factor VII (VIIa) forming a complex that activates thrombus formation. TF is expressed on vascular endothelial cells, circulating monocyte and platelet where its activation upregulates VEGF that promotes angiogenesis. Non-genomic actions of thyroid hormones are initiated on integrin αvβ3. Thyroid hormones activate the NF-kB pathway, thus promoting the production of VEGF, cell proliferation and angiogenesis. Thrombus formation and angiogenesis stimulation affect inflammation pathway and vice versa. Either sulfated non-anticoagulant LMWH (SNACH) or integrin αvβ3 receptor blockade suppresses inflammation, angiogenesis, and indirectly the thrombosis processes. abbreviations: ABP-280, actin-binding protein 280; NF-KB, nuclear factor kappa B; TF, tissue factor; TFPI, tissue factor pathway inhibitor; TREs, thyroid hormone response elements; TRs, thyroid hormone receptors. (Modified with permission from Mousa SA. Heparin and low-molecular weight heparins in thrombosis and beyond. Methods Mol Biol 2010;663:109-132.).
Microvesicles
A heterogeneous population of small membrane-enclosed structures, EVs are released into the extracellular space. They have been divided into 3 main categories according to their biogenesis; exosomes, microvesicles (MVs) and apoptotic bodies.91 The diversity of MVs’ origin is responsible for a variety of structures and composition in lipids, proteins and nucleic acids and thus MVs are involved in different pathophysiological processes such as coagulation, inflammation, angiogenesis or endothelial dysfunction.92 MVs have to be integrated in their complex vascular environment with all partners (innate immune cells such as polymorphonuclear cells, monocytes, endothelial cells, platelets, and tumor cells) contributing to thrombus formation.93
Endothelial
Although TF is widely expressed on subendothelial cells such as fibroblasts, pericytes, and vascular smooth muscle cells, it is not expressed in normal endothelium; malignant tissue involving endothelial and tumor cells express TF constitutively.94 Platelets and endothelia cells have p-selectin on their surfaces.95 P-selectin could increase VTE through leukocyte recruitment.96
Neutrophil extracellular traps
Cancer cells may release NETs97 and they have been shown to endorse venous and arterial thrombosis.98 NETs can activate endothelial cells and eventually increase the release of von Willebrand factor (a significant glycoprotein for platelet adherence and thrombosis aggregation)98 and serve as a platform to adhere and aggregate platelets,99 which is vital for thrombus formation.100 Cancers create a systemic environment that prevents NETs from releasing neutrophils that modulate different facets of the tumor biology, including metastasis.101
Cancer procoagulant
Cancer procoagulant (CP) is unlike TF in that it directly activates factor X independent of coagulation factor VII. It has been detected in different malignant cells such as leukemia and breast cancer and is present in solid and hematologic tumors but not in normal tissues.102
Monocytes
Monocytes and macrophages express TF in an inactive form and then specific inflammatory agonist pathways convert it to totally procoagulant TF. Activation of TF on monocytes or macrophages requires an exchange of thiol-disulfide and involves protein disulfide isomerase (PDI). While the PDI can be released during vessel wall injury,103 in vitro research showed that PDI is present on the surface of intact cells104 in complex with TF105 and PDI-regulated TF activity on EVs.106
Cytokines
Tumor cells release cytokines into the bloodstream. Leukocytes and vascular cells are both cytokine sources and targets for them. Hemostatic balance depends on the complex interactions between endothelial cells, blood cells, the coagulation-fibrinolytic system, and cytokines.107 Pro-inflammatory cytokines such as interleukin-1 (IL-1), IL-6, and tumor necrosis factor (TNF) induce TF in monocytes.108,109 Such cytokines not only trigger procoagulant activity but also impede the anticoagulation pathway of thrombomodulin/protein C and affect fibrinolysis by upregulating urokinase type plasminogen activator and type I inhibitor of such activation.107 Indeed, cytokine production is significantly increased in cancer patients by activating host cells, like monocytes and endothelial cells as well as by cytokine release itself through tumor cells.110 Cytokines like IFN-α, IFN-γ and TNF can boost the activity of tumor cell procoagulants and consequently activate clotting in patients with cancer.111
Current Management of CAT
Low-molecular-weight heparin
LMWH inhibits coagulation by activating antithrombin III, which binds to and inhibits factor Xa and IIa.112 In the absence of contra-indications, LMWH is preferred over vitamin K antagonists (VKA) for the treatment of VTE in patients with cancer based on 4 previous studies that showed the high efficacy and superiority of LMWHs compared to VKAs.113–116 First, the Randomized Comparison of Low-Molecular-Weight Heparin Versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) study compared dalteparin (LMWH) to warfarin and showed a 55% reduction in relative risk of recurrent VTE without increasing major bleeding.114 Another study is the Comparison of Acute Treatments in Cancer Haemostasis (CATCH) trial that compared LMWH (tinzaparin) to warfarin and showed a trend toward reduction in VTE recurrence, but the difference was not statistically significant. Tinzaparin showed a safer profile in clinically relevant nonmajor bleeding than warfarin.116 The other 2 studies confirmed that warfarin is associated with a high bleeding rate in patients with CAT.113,115 Therefore, in CAT, LMWH is preferred in VTE prophylaxis and treatment.
Direct oral anticoagulants (DOACs)
Four DOACs, including dabigatran (direct thrombin inhibitor), rivaroxaban, apixaban, and edoxaban (direct factor Xa inhibitor), have been approved by the FDA for VTE therapy. They have benefits over VKAs including safety such as a lower incidence of major bleeding, more convenient to use, fewer drug and food interactions, a wide therapeutic index, and there is no periodic laboratory tracking required. Furthermore, unlike LMWHs, DOACs are taken orally and don’t require long-term subcutaneous injections.117
Previous randomized clinical trials compared DOACs and LMWHs in the treatment of CAT, such as the Hokusai VTE Cancer,118 SELECT-D,119 and ADAM VTE120 trials. They reported that DOACs are not inferior to LMWHs for preventing VTE recurrence in patients with cancer. The Hokusai VTE trial and SELECT-D trial revealed that DOACs were non-inferior to subcutaneous LMWH, and the rate of recurrent VTE was lower, but the rate of major bleeding was higher with edoxaban (DOAC) than with dalteparin (LMWH).118,119 The SELECT-D study reported significant bleeding events as a side effect to DOACs.119 In patients treated with rivaroxaban (DOACs), more clinically relevant non-major bleeding did happen. Most bleeds were gastrointestinal, and the distinction in bleeding risk was most apparent for gastrointestinal cancer patients.118 Recently, a meta-analysis study reported that the risk of VTE recurrence in patients with cancer receiving DOACs as compared to LMWHs is reduced but with an increased number of episodes of major bleeding.121 Recently the results of the Caravaggio Investigators’ study showed that there was no difference between treatment with DOACs and LMWHs in the number of CAT relapses over a 6-month period. Moreover, it showed that in patients less than 65 years of age, apixaban prevented the recurrence of VTE more than dalteparin and the effect of apixaban was decreased in elderly patients. There were also no differences in the risk of major gastrointestinal bleeding and total mortality although the number of clinically relevant non-major bleeding episodes was higher after apixaban use.122 Table 1 summarizes data with LMWH versus warfarin and DOAC in CAT.
Table 1.
Comparison Between Low-Molecular-Weight Heparin (LMWH) Versus Other Anticoagulants for Treatment of VTE in Patients With Cancer.
| Study | Months | No. of enrolled patients | Treatment arms | Outcome |
|---|---|---|---|---|
| CLOT114 | 6 | 672 | Dalteparin versus warfarin | Dalteparin superior to warfarin in VTE recurrence (P = 0.002). No difference in major bleeding or mortality. |
| CATCH116 | 6 | 900 | Tinzaparin versus warfarin | Statistical tendency of VTE recurrence reduction with tinzaparin. No difference in major bleeding or mortality. |
| CANTHANOX113 | 3 | 138 | Enoxaparin versus warfarin | Warfarin associated with high bleeding rate in patients with VTE and cancer. Prolonged treatment with LMWHs may be as effective as oral anticoagulants and may be safer in these patients. |
| Hull et al.115 | 12 | 200 | Tinzaparin versus warfarin | Long-term LMWHs more effective than vitamin K antagonist therapy for preventing recurrent VTE in patients with cancer and proximal venous thrombosis. Usual-care group had excess of recurrent VTE. |
| Hokusai Cancer VTE118 | 12 | 1046 | Edoxaban versus dalteparin | Edoxaban was non-inferior to dalteparin. Statistical tendency of superiority of edoxaban in VTE recurrence reduction, more major bleeding with edoxaban. |
| SELECT-D119 | 6 | 406 | Rivaroxaban versus dalteparin | Less VTE recurrence (HR 0.43, 95% CI 0.19–0.99) and statistical tendency of more bleeding (HR 1.83, 95% CI 0.68–4.96) with rivaroxaban. |
| ADAM-VTE120 | 6 | 300 | Apixaban versus dalteparin | Primary efficacy outcome, recurrent symptomatic VTE or death related to VTE, occurred in 2.3% in apixaban group and 2.7% in conventional therapy group. From an efficacy standpoint, apixaban considered non-inferior to standard anticoagulant therapy. Major bleeding significantly lower for patients receiving apixaban. |
| Caravaggio Investigators122 | 6 | 1170 | Apixaban versus dalteparin |
Recurrent VTE occurred in 5.6% in apixaban group and 7.9% in the dalteparin group (P < 0.001) for non-inferiority). Major bleeding occurred in 3.8% in apixaban group and 4.0% in dalteparin group. |
Future Directions in the Management of CAT
Euthyroid
Several studies have reported an increased thrombophilic risk profile in patients with overt hyperthyroidism and sub-clinical hyperthyroidism,; this transforms the hemostatic balance through hypercoagulable and hypofibrinolytic state with elevated levels of factor VIII, factor IX, von Willebrand factor antigen (VWF: Ag), fibrinogen, and plasminogen activator inhibitor.123 The evidence suggests that hypothyroidism may have a biphasic effect on fibrinolysis, depending on disease severity.124 A previous study found that T3 may directly or indirectly control transcription of hepatic and vessel walls’ coagulation; this transcriptional modification resulted in a change in the plasma coagulation factors,125 Moreover, spontaneous or medically induced hypothyroidism may alter the clinical behavior of cancers such as breast cancer,126 glioblastoma127 and renal cell carcinoma.128 T3 reduces serum thyrotropin levels (TSH) and T4 level and also improves clinical and radiological conditions in cancer patients.129 In fact, T3 cannot induce tumor cell proliferation at physiological concentrations.130 When the T3 level increases, it can stimulate cancer cell proliferation at the receptor on integrin αvβ3.131 Nevertheless, in patients with euthyroidism and cancer, euthyroidism alone with a normal T3 level does not seem to promote the growth of the tumor.130 T4 has a significant action on tumor cells and coagulation when compared to T3. This may represent a novel, inexpensive and nontoxic approach for improving CAT and TAC. The paradigm should be tested in prospective controlled trials in a wider variety of patients with cancer. Thus future studies are needed to investigate the effect of euthyroid in CAT and TAC.
Blockade of thyroid hormone receptors on integrin αvβ3
The plasma membrane integrin αvβ3 acts as a membrane receptor for thyroid hormone. Integrin αvβ3 binding facilitates the hormone’s proliferative action on cancer cells and blood vessel cells.131 The primary thyroid hormone ligand of this receptor is T4, which activates β-catenin and induces metastasis.132 The activation and deactivation of thyroid hormones may also stimulate angiogenesis associated with primary or metastatic tumors. As stated above, various mechanisms stimulate angiogenesis among the non-genomic actions of T4 and T3 at αvβ3, of tumor cell proliferation, and of anti-apoptotic tumor defenses.133, These problems may be avoided by using an analog of T4, tetraiodothyroacetic acid (tetrac), which prevents binding of iodothyronines to integrin αvβ3.134 In order to limit tetrac to the cell surface of thyroid hormone receptor, diamino propane tetraiodothyroacetic acid (DAT) is preferred. Tetrac and DAT as thyroid hormone derivatives influence gene expression after crossing cellular membranes. We conjugated tetrac to the polymeric nanoparticle poly (lactic-co-glycolic acid) (PLGA) resulting in nano-diamino-tetrac (NDAT).135 Many studies have shown that the formulation of NDAT is restricted to the extracellular area and to the integrin, thus slowing tumor development and vascularity.136 NDAT’s antitumor efficacy includes pro-apoptotic activity and gene transcription disturbance that are crucial to a number of mechanisms for cancer cell survival.137 Tetrac blocks the pro-angiogenic activity of VEGF and of basic fibroblast growth factor (bFGF; FGF2) in the chick chorioallantoic membrane and in an endothelial cell microtubule formation assay138 and PDGF.51 Moreover, tetrac prevents radioresistance of cancer cells resulting from the activation of integrin αvβ3.139 Another drug that can block thyroid hormone on integrin αvβ3 is compound XT199, which is an integrin antagonist that can block aggregatory effects on platelets. It acts at the cell surface via interaction with integrin αvβ3 and thyroid hormone receptors.140
SNACH (Sulfated non-anticoagulant heparin)
It was shown that the effect of SNACH in inhibiting cancer metastasis is not related to the heparin anticoagulant property.136 SNACH is a LMWH that has no effect on the systemic coagulation factors yet releases TF pathway inhibitor protein (TFPI), a key endogenous inhibitor of the TF/VIIa complex from the endothelium141 (Figure 2). Release of TFPI may be responsible for the anti-angiogenic and antimetastatic activity. Like most of the non-antithrombin-mediated effects, TFPI release from the vascular endothelium is associated with N-sulfated regions of heparin and non-anticoagulant heparins.141 The additional finding that SNACH exhibits anti-angiogenesis activity suggests another mechanism that could contribute to its role in tumor suppression.141 As shown in Figure 2, it can be concluded that SNACH releases TFPI that affects inflammation and angiogenesis, and it has an inhibitory effect on TF/VIIa complex that affects activation of coagulation and platelets and consequently affects CAT.
Inhibition of NETs
In cancer, NETs circulate at high levels in blood,142 activate the occlusion of small vessels,143 activate the contact pathway of coagulation,144 and help in digesting the major coagulation inhibitors such as antithrombin III and TFPI.145 Dissolving NETs with DNase I restores normal perfusion of microvasculature in animal models.143 Also, DNase I proved its significance in inhibiting tumorigenicity of some cancer cell lines.146 There are many drugs that can be used as NET inhibitors by reducing NETs’ formation such as peptidyl arginine deiminase type 4 inhibitors,147 cyclooxygenase-2148 and vitamin C.149 Based on the above findings, targeting intravascular NETs may similarly reduce thrombosis in patients with cancer.
Risk stratification strategies
Prevention and treatment of CAT can be achieved by a variety of risk stratification strategies. Risk factors are either patient-related factors, cancer-related factors, or treatment-related factors.150 The use of single clinical risk factors as a risk stratification failed. Therefore, the new American Society of Clinical Oncology (ASCO) guidelines recommend the use of a risk score that includes multiple variables to identify high-risk patients151,152 such as elevated platelet and leukocyte counts, decreased hemoglobin, elevated D-dimer, elevated prothrombin activation products, elevated soluble P-selectin, peak thrombin generation, and elevated levels of TF-bearing microparticles.153 Potential applications of risk factors stratification include educating patients about the warning signs and symptoms of VTE, which could lead to early detection and treatment154 and targeted prophylaxis that supports the “precision medicine” approach that is the hallmark of anticancer therapy.155
The Link between CAT and TAC
The strong link between cancer and thrombosis is illustrated in Figure 1 along with CAT and TAC. Tumor cells may activate the coagulation system and lead to thrombosis through the production of procoagulant, fibrinolytic, and pro-inflammatory and pro-angiogenic cytokines, leading to the prothrombotic state and tumor metastasis in patients with cancer.156
On the other hand, coagulation factors also affect the progression of cancer leading to TAC. Previous studies suggested that hemostatic abnormalities in patients with cancer can contribute to inflammatory cell recruitment, tumor stroma production, and angiogenesis.157 We know that TF is expressed on cancer cells and vasculature158 as well as on endothelial cells and platelets, and TF-bearing EVs contribute to the thrombotic phenotype in cancer patients.159 The TF/factor VIIa complex has an effect on processes of blood coagulation and has an effect on inflammation and angiogenesis.160 Higher TFPI-1 levels may be due to the TF uptake in tumor cells or to damaged endothelial cells in ongoing coagulation activation in patients with cancer and DVT or metastases.161 TF also regulates αvβ3 integrin, which as stated earlier, is expressed on platelets, cancer cells, and endothelium. Integrin αvβ3 activation facilitates tumor angiogenesis and tumor progression.162 In summary, TF isoforms, factor VII, PAR2, and αvβ3 integrin affect cell process and cell interaction with their environment, including angiogenic events.163
Conclusion
There is a two-way clinical relationship between cancer and VTE. Thrombotic events, especially idiopathic thrombosis, can sometimes be a cause of cancer. At the same time, VTE is a serious complication of cancer and the second most frequent cause of death in patients with cancer. Moreover, the prevention and treatment of VTE in cancer patients is challenging because the risk-benefit profile when using anticoagulants must be considered. Further data are needed to confirm future directions in the management of CAT or TAC by either euthyroid, blockade of thyroid hormone on integrin αvβ3 by NDAT or other agents, by sulfated non-anticoagulant heparin (SNACH), inhibition of NETs, stratifying low and high risk patients with significant bleeding problems with DOACs, or other appropriate therapies.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Shaker A. Mousa  https://orcid.org/0000-0002-9294-015X
https://orcid.org/0000-0002-9294-015X
References
- 1. Sack GH, Jr, Levin J, Bell WR. Trousseau’s syndrome and other manifestations of chronic disseminated coagulopathy in patients with neoplasms: clinical, pathophysiologic, and therapeutic features. Medicine (Baltimore). 1977;56(1):1–37. [PubMed] [Google Scholar]
- 2. ISTH Steering Committee for World Thrombosis Day. Thrombosis: a major contributor to global disease burden. Thromb Res. 2014;134(5):931–938. [DOI] [PubMed] [Google Scholar]
- 3. Imberti D, Agnelli G, Ageno W, et al. Clinical characteristics and management of cancer-associated acute venous thromboembolism: findings from the MASTER registry. Haematologica. 2008;93(2):273–278. [DOI] [PubMed] [Google Scholar]
- 4. Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5(3):632–634. [DOI] [PubMed] [Google Scholar]
- 5. Braekkan SK, Borch KH, Mathiesen EB, Njolstad I, Wilsgaard T, Hansen JB. . Body height and risk of venous thromboembolism: the tromso study. Am. J. Epidemiol. 2010;171(10):1109–1115. [DOI] [PubMed] [Google Scholar]
- 6. Elyamany G, Alzahrani AM, Bukhary E. Cancer-associated thrombosis: an overview. Clin Med Insights Oncol. 2014;8:129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Puurunen MK, Gona PN, Larson MG, Murabito JM, Magnani JW, O’Donnell CJ. Epidemiology of venous thromboembolism in the Framingham heart study. Thromb Res. 2016;145:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122(10):1712–1723. [DOI] [PubMed] [Google Scholar]
- 9. Cronin-Fenton DP, Sondergaard F, Pedersen LA, et al. Hospitalisation for venous thromboembolism in cancer patients and the general population: a population-based cohort study in Denmark, 1997-2006. Br J Cancer. 2010;103(7):947–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Walker AJ, Card TR, West J, Crooks C, Grainge MJ. Incidence of venous thromboembolism in patients with cancer – a cohort study using linked United Kingdom databases. Eur J Cancer. 2013;49(6):1404–1413. [DOI] [PubMed] [Google Scholar]
- 11. Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293(6):715–722. [DOI] [PubMed] [Google Scholar]
- 12. Secin FP, Jiborn T, Bjartell AS, et al. Multi-institutional study of symptomatic deep venous thrombosis and pulmonary embolism in prostate cancer patients undergoing laparoscopic or robot-assisted laparoscopic radical prostatectomy. Eur Urol. 2008;53(1):134–145. [DOI] [PubMed] [Google Scholar]
- 13. Wun T, White RH. Epidemiology of cancer-related venous thromboembolism. Best Pract Res Clin Haematol. 2009;22(1):9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ikushima S, Ono R, Fukuda K, Sakayori M, Awano N, Kondo K. Trousseau’s syndrome: cancer-associated thrombosis. Jpn J Clin Oncol. 2016;46(3):204–208. [DOI] [PubMed] [Google Scholar]
- 15. Nasser NJ, Fox J, Agbarya A. Potential mechanisms of cancer-related hypercoagulability. Cancers (Basel). 2020;12(3):566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wall JG, Weiss RB, Norton L, et al. Arterial thrombosis associated with adjuvant chemotherapy for breast carcinoma: a cancer and leukemia group B study. Am J Med. 1989;87(5):501–504. [DOI] [PubMed] [Google Scholar]
- 17. Blom JW, Vanderschoot JP, Oostindier MJ, Osanto S, van der Meer FJ, Rosendaal FR. Incidence of venous thrombosis in a large cohort of 66,329 cancer patients: results of a record linkage study. J Thromb Haemost. 2006;4(3):529–535. [DOI] [PubMed] [Google Scholar]
- 18. Haddad TC, Greeno EW. Chemotherapy-induced thrombosis. Thromb. Res. 2006;118(5):555–568. [DOI] [PubMed] [Google Scholar]
- 19. Cool RM, Herrington JD, Wong L. Recurrent peripheral arterial thrombosis induced by cisplatin and etoposide. Pharmacotherapy. 2002;22(9):1200–1204. [DOI] [PubMed] [Google Scholar]
- 20. Mannucci PM, Bettega D, Chantarangkul V, Tripodi A, Sacchini V, Veronesi U. Effect of tamoxifen on measurements of hemostasis in healthy women. Arch Intern Med. 1996;156(16):1806–1810. [PubMed] [Google Scholar]
- 21. Mills PJ, Parker B, Jones V, et al. The effects of standard anthracycline-based chemotherapy on soluble ICAM-1 and vascular endothelial growth factor levels in breast cancer. Clin Cancer Res. 2004;10(15):4998–5003. [DOI] [PubMed] [Google Scholar]
- 22. Walsh J, Wheeler HR, Geczy CL. Modulation of tissue factor on human monocytes by cisplatin and Adriamycin. Br J Haematol. 1992;81(4):480–488. [DOI] [PubMed] [Google Scholar]
- 23. Lechner D, Kollars M, Gleiss A, Kyrle PA, Weltermann A. Chemotherapy-induced thrombin generation via procoagulant endothelial microparticles is independent of tissue factor activity. J Thromb Haemost. 2007;5(12):2445–2452. [DOI] [PubMed] [Google Scholar]
- 24. Bezeaud A, Drouet L, Leverger G, Griffin JH, Guillin MC. Effect of L-asparaginase therapy for acute lymphoblastic leukemia on plasma vitamin K-dependent coagulation factors and inhibitors. J Pediatr. 1986;108(5 Pt 1):698–701. [DOI] [PubMed] [Google Scholar]
- 25. Sibai H, Chen R, Liu X, et al. Anticoagulation prophylaxis reduces venous thromboembolism rate in adult acute lymphoblastic leukaemia treated with asparaginase-based therapy. Br J Haematol. 2020; Epub May 13 doi:10.1111/bjh.16695. [DOI] [PubMed] [Google Scholar]
- 26. Feffer SE, Carmosino LS, Fox RL. Acquired protein C deficiency in patients with breast cancer receiving cyclophosphamide, methotrexate, and 5-fluorouracil. Cancer. 1989;63(7):1303–1307. [DOI] [PubMed] [Google Scholar]
- 27. Koster T, Rosendaal FR, de Ronde H, Briet E, Vandenbroucke JP, Bertina RM. Venous thrombosis due to poor anticoagulant response to activated protein C: Leiden thrombophilia study. Lancet. 1993;342(8886-8887):1503–1506. [DOI] [PubMed] [Google Scholar]
- 28. Liberti G, Bertina RM, Rosendaal FR. Hormonal state rather than age influences cut-off values of protein S: reevaluation of the thrombotic risk associated with protein S deficiency. Thromb Haemost. 1999;82(3):1093–1096. [PubMed] [Google Scholar]
- 29. Giustozzi M, Curcio A, Weijs B, et al. Variation in the association between antineoplastic therapies and venous thromboembolism in patients with active cancer. Thromb Haemost. 2020;120(5):847–856. [DOI] [PubMed] [Google Scholar]
- 30. Maraveyas A, Haque F, Muazzam IA, Ilyas W, Bozas G. Increased dose primary thromboprophylaxis in ambulatory patients with advanced pancreatic ductal adenocarcinoma, a single centre cohort study. Thromb J. 2020;18:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Carmona-Bayonas A, Gómez D, Martínez de Castro E, et al. A snapshot of cancer-associated thromboembolic disease in 2018-2019: first data from the TESEO prospective registry. Eur J Intern Med. 2020;78:41–49. [DOI] [PubMed] [Google Scholar]
- 32. Guy JB, Falk AT, Chargari C, Bertoletti L, Magne N. Thromboembolic events following brachytherapy: case reports. J Contemp Brachytherapy. 2015;7(1):76–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guy JB, Bertoletti L, Magne N, et al. Venous thromboembolism in radiation therapy cancer patients: findings from the RIETE registry. Crit Rev Oncol Hematol. 2017;113:83–89. [DOI] [PubMed] [Google Scholar]
- 34. Geiger H, Pawar SA, Kerschen EJ, et al. Pharmacological targeting of the thrombomodulin-activated protein C pathway mitigates radiation toxicity. Nat Med. 2012;18(7):1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Byrne M, Reynolds JV, O’Donnell JS, et al. Long-term activation of the pro-coagulant response after neoadjuvant chemoradiation and major cancer surgery. Br J Cancer. 2010;102(1):73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Boerma M, Kruse JJ, van Loenen M, et al. Increased deposition of von Willebrand factor in the rat heart after local ionizing irradiation. Strahlenther Onkol. 2004;180(2):109–116. [DOI] [PubMed] [Google Scholar]
- 37. Leith JT, Hercbergs A, Kenney S, Mousa SA, Davis PJ. Activation of tumor cell integrin αvβ3 by radiation and reversal of activation by chemically modified tetraiodothyroacetic acid (tetrac). Endocr Res. 2018;43(4):215–219. [DOI] [PubMed] [Google Scholar]
- 38. Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–333. [DOI] [PubMed] [Google Scholar]
- 39. Mandala M, Falanga A, Roila F. Management of venous thromboembolism (VTE) in cancer patients: ESMO clinical practice guidelines. Ann Oncol. 2011;22(Suppl 6):vi85–92. [DOI] [PubMed] [Google Scholar]
- 40. Horlander KT, Mannino DM, Leeper KV. Pulmonary embolism mortality in the United States, 1979-1998: an analysis using multiple-cause mortality data. Arch. Intern. Med. 2003;163(14):1711–1717. [DOI] [PubMed] [Google Scholar]
- 41. Jordan BJ, Matulewicz RS, Trihn B, Kundu S. Venous thromboembolism after nephrectomy: incidence, timing and associated risk factors from a national multi-institutional database. World J Urol. 2017;35(11):1713–1719. [DOI] [PubMed] [Google Scholar]
- 42. Edelman JJ, Reddel CJ, Kritharides L, et al. Natural history of hypercoagulability in patients undergoing coronary revascularization and effect of preoperative myocardial infarction. J Thorac Cardiovasc Surg. 2014;148(2):536–543. [DOI] [PubMed] [Google Scholar]
- 43. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e227S–e277S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bohlius J, Wilson J, Seidenfeld J, et al. Recombinant human erythropoietins and cancer patients: updated meta-analysis of 57 studies including 9353 patients. J Natl Cancer Inst. 2006;98(10):708–714. [DOI] [PubMed] [Google Scholar]
- 45. Bennett CL, Silver SM, Djulbegovic B, et al. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA. 2008;299(8):914–924. [DOI] [PubMed] [Google Scholar]
- 46. Smith KJ, Bleyer AJ, Little WC, Sane DC. The cardiovascular effects of erythropoietin. Cardiovasc Res. 2003;59(3):538–548. [DOI] [PubMed] [Google Scholar]
- 47. Fuste B, Serradell M, Escolar G, et al. Erythropoietin triggers a signaling pathway in endothelial cells and increases the thrombogenicity of their extracellular matrices in vitro. Thromb Haemost. 2002;88(4):678–685. [PubMed] [Google Scholar]
- 48. Ortiga Carvalho TM, Chiamolera MI, Pazos Moura CC, Wondisford FE. Hypothalamus-pituitary-thyroid axis. Compr Physiol. 2016;6(3):1387–1428. [DOI] [PubMed] [Google Scholar]
- 49. Hellevik AI, Asvold BO, Bjoro T, Romundstad PR, Nilsen TI, Vatten LJ. Thyroid function and cancer risk: a prospective population study. Cancer Epidemiol Biomarkers Prev. 2009;18(2):570–574. [DOI] [PubMed] [Google Scholar]
- 50. Davis PJ, Davis FB, Mousa SA, Luidens MK, Lin HY. Membrane receptor for thyroid hormone: physiologic and pharmacologic implications. Annu Rev Pharmacol Toxicol. 2011;51:99–115. [DOI] [PubMed] [Google Scholar]
- 51. Davis PJ, Glinsky GV, Lin HY, et al. Cancer cell gene expression modulated from plasma membrane integrin αvβ3 by thyroid hormone and nanoparticulate tetrac. Front Endocrinol (Lausanne). 2014;5:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Brent GA. Mechanisms of thyroid hormone action. J Clin Invest. 2012;122(9):3035–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cayrol F, Sterle HA, Díaz Flaqué MC, Barreiro Arcos ML, Cremaschi GA. Non-genomic actions of thyroid hormones regulate the growth and angiogenesis of T cell lymphomas. Front Endocrinol. 2019;10:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bergh JJ, Lin HY, Lansing L, et al. Integrin avb3 contains a cell surface receptor site for thyroid hormone that is linked to activation of mitogen-activated protein kinase and induction of angiogenesis. Endocrinology. 2005;146(7):2864–2871. [DOI] [PubMed] [Google Scholar]
- 55. Davis PJ, Davis FB, Mousa SA. Thyroid hormone-induced angiogenesis. Curr Cardiol Rev. 2009;5(1):12–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mousa SS, Davis FB, Davis PJ, Mousa SA. Human platelet aggregation and degranulation is induced in vitro by L-thyroxine, but not by 3,5,3’-triiodo-L-thyronine or diiodothyropropionic acid (DITPA). Clin Appl Thromb Hemost. 2010;16(3):288–293. [DOI] [PubMed] [Google Scholar]
- 57. Meikle CKS, Kelly CA, Garg P, Wuescher LM, Ali RA, Worth RG. Cancer and thrombosis: the platelet perspective. Front Cell Dev Biol. 2017;4:147–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and estrogen/progestin replacement study (HERS) research group. JAMA. 1998;280(7):605–613. [DOI] [PubMed] [Google Scholar]
- 59. Bharti JN, Rani P, Kamal V, Agarwal PN. Angiogenesis in breast cancer and its correlation with estrogen, progesterone receptors and other prognostic factors. J Clin Diagn Res. 2015;9(1): Ec05–07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhang Y, Hua F, Ding K, Chen H, Xu C, Ding W. Angiogenesis changes in ovariectomized rats with osteoporosis treated with estrogen replacement therapy. BioMed res int. 2019;2019:1283717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20(3):358–417. [DOI] [PubMed] [Google Scholar]
- 62. Jayachandran M, Miller VM. Human platelets contain estrogen receptor a, caveolin-1 and estrogen receptor associated proteins. Platelets. 2003;14(2):75–81. [DOI] [PubMed] [Google Scholar]
- 63. Sar P, Peter R, Rath B, Das Mohapatra A, Mishra SK. 3, 3’5 Triiodo L thyronine induces apoptosis in human breast cancer MCF-7 cells, repressing SMP30 expression through negative thyroid response elements. PLoS One. 2011;6(6):e20861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Andersen CL, Sikora MJ, Boisen MM, et al. Active estrogen receptor-alpha signaling in ovarian cancer models and clinical specimens. Clin Cancer Res. 2017;23(14):3802–3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rak J, Milsom C, May L, Klement P, Yu J. Tissue factor in cancer and angiogenesis: the molecular link between genetic tumor progression, tumor neovascularization, and cancer coagulopathy. Semin Thromb Hemost. 2006;32(1):54–70. [DOI] [PubMed] [Google Scholar]
- 66. Ancrile B, Lim KH, Counter CM. Oncogenic Ras-induced secretion of IL6 is required for tumorigenesis. Genes Dev. 2007;21(14):1714–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chennakrishnaiah S, Meehan B, D’Asti E, et al. Leukocytes as a reservoir of circulating oncogenic DNA and regulatory targets of tumor-derived extracellular vesicles. J Thromb Haemost. 2018;16(9):1800–1813. [DOI] [PubMed] [Google Scholar]
- 68. Garnier D, Magnus N, Lee TH, et al. Cancer cells induced to express mesenchymal phenotype release exosome-like extracellular vesicles carrying tissue factor. J Biol Chem. 2012;287(52):43565–43572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yu JL, May L, Lhotak V, et al. Oncogenic events regulate tissue factor expression in colorectal cancer cells: implications for tumor progression and angiogenesis. Blood. 2005;105(4):1734–1741. [DOI] [PubMed] [Google Scholar]
- 70. Strömblad S, Becker JC, Yebra M, Brooks PC, Cheresh DA. Suppression of p53 activity and p21WAF1/CIP1 expression by vascular cell integrin alphaVbeta3 during angiogenesis. J Clin Invest. 1996;98(2):426–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Rak J, Klement G. Impact of oncogenes and tumor suppressor genes on deregulation of hemostasis and angiogenesis in cancer. Cancer Metastasis Rev. 2000;19(1-2):93–96. [DOI] [PubMed] [Google Scholar]
- 72. Tape CJ, Ling S, Dimitriadi M, et al. Oncogenic KRAS regulates tumor cell signaling via stromal reciprocation. Cell. 2016;165(4):910–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Spranger S, Gajewski TF. Impact of oncogenic pathways on evasion of antitumour immune responses. Nat rev Cancer. 2018;18(3):139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Unruh D, Schwarze SR, Khoury L, et al. Mutant IDH1 and thrombosis in gliomas. Acta Neuropathol. 2016;132(6):917–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yu JL, Xing R, Milsom C, Rak J. Modulation of the oncogene-dependent tissue factor expression by kinase suppressor of Ras 1. Thromb Res. 2010;126(1):e6–10. [DOI] [PubMed] [Google Scholar]
- 76. Xu XR, Zhang D, Oswald BE, et al. Platelets are versatile cells: new discoveries in hemostasis, thrombosis, immune responses, tumor metastasis and beyond. Crit Rev Clin. Lab Sci. 2016;53(6):409–430. [DOI] [PubMed] [Google Scholar]
- 77. Schlesinger M. Role of platelets and platelet receptors in cancer metastasis. J Hematol Oncol. 2018;11(1):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. De Candia E. Mechanisms of platelet activation by thrombin: a short history. Thromb Res. 2012;129(3):250–256. [DOI] [PubMed] [Google Scholar]
- 79. Menter DG, Tucker SC, Kopetz S, Sood AK, Crissman JD, Honn KV. Platelets and cancer: a casual or causal relationship: revisited. Cancer Metastasis Rev. 2014;33(1):231–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lechner D, Weltermann A. Chemotherapy-induced thrombosis: a role for microparticles and tissue factor? Semin Thromb Hemost. 2008;34(2):199–203. [DOI] [PubMed] [Google Scholar]
- 81. Gremmel T, Frelinger AL, Michelson AD. Platelet physiology. Semin Thromb Hemost. 2016;42(3):191–204. [DOI] [PubMed] [Google Scholar]
- 82. Li N. Platelets in cancer metastasis: to help the “villain” to do evil. Int j cancer. 2016;138(9):2078–2087. [DOI] [PubMed] [Google Scholar]
- 83. Wojtukiewicz MZ, Sierko E, Hempel D, Tucker SC, Honn KV. Platelets and cancer angiogenesis nexus. Cancer Metastasis Rev. 2017;36(2):249–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hoffman M, Monroe DM. A cell-based model of hemostasis. Thromb Haemost. 2001;85(6):958–965. [PubMed] [Google Scholar]
- 85. Palta S, Saroa R, Palta A. Overview of the coagulation system. Indian J Anaesth. 2014;58(5):515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Aleman MM, Byrnes JR, Wang JG, et al. Factor XIII activity mediates red blood cell retention in venous thrombi. J Clin Invest. 2014;124(8):3590–3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Duvernay M, Young S, Gailani D, Schoenecker J, Hamm HE. Protease-activated receptor (PAR) 1 and PAR4 differentially regulate factor V expression from human platelets. Mol Pharmacol. 2013;83(4):781–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Neuenschwander PF, Fiore MM, Morrissey JH. Factor VII autoactivation proceeds via interaction of distinct protease-cofactor and zymogen-cofactor complexes. Implications of a two-dimensional enzyme kinetic mechanism. J Biol Chem. 1993;268(29):21489–21492. [PubMed] [Google Scholar]
- 89. Versteeg HH, Heemskerk JW, Levi M, Reitsma PH. New fundamentals in hemostasis. Physiol Rev. 2013;93(1):327–358. [DOI] [PubMed] [Google Scholar]
- 90. Ettelaie C, Collier ME, Featherby S, Benelhaj NE, Greenman J, Maraveyas A. Analysis of the potential of cancer cell lines to release tissue factor-containing microvesicles: correlation with tissue factor and PAR2 expression. Thromb J. 2016;14:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Boulanger CM, Loyer X, Rautou PE, Amabile N. Extracellular vesicles in coronary artery disease. Nat Rev Cardiol. 2017;14(5):259–272. [DOI] [PubMed] [Google Scholar]
- 92. Ridger VC, Boulanger CM, Scherrer AA, et al. Microvesicles in vascular homeostasis and diseases. Position paper of the European society of cardiology (esc) working group on atherosclerosis and vascular biology. Thromb Haemost. 2017;117(7):1296–1316. [DOI] [PubMed] [Google Scholar]
- 93. Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013;13(1):34–45. [DOI] [PubMed] [Google Scholar]
- 94. Abdol Razak NB, Jones G, Bhandari M, Berndt MC, Metharom P. Cancer-associated thrombosis: an overview of mechanisms, risk factors, and treatment. Cancers (Basel). 2018;10(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ay C, Simanek R, Vormittag R, et al. High plasma levels of soluble P-selectin are predictive of venous thromboembolism in cancer patients: results from the Vienna cancer and thrombosis study (CATS). Blood. 2008;112(7):2703–2708. [DOI] [PubMed] [Google Scholar]
- 96. Meier TR, Myers DD, Jr, Wrobleski SK, et al. Prophylactic P-selectin inhibition with PSI-421 promotes resolution of venous thrombosis without anticoagulation. Thromb Haemost. 2008;99(2):343–351. [DOI] [PubMed] [Google Scholar]
- 97. Abdol Razak N, Elaskalani O, Metharom P. Pancreatic cancer-induced neutrophil extracellular traps: a potential contributor to cancer-associated thrombosis. Int J Mol Sci. 2017;18(3):487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. von Bruhl ML, Stark K, Steinhart A, et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012;209(4):819–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. McDonald B, Davis RP, Kim SJ, et al. Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood. 2017;129(10):1357–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Mauracher LM, Posch F, Martinod K, et al. Citrullinated histone H3, a biomarker of neutrophil extracellular trap formation, predicts the risk of venous thromboembolism in cancer patients. J Thromb Haemost. 2018;16(3):508–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Demers M, Wagner DD. Neutrophil extracellular traps: a new link to cancer-associated thrombosis and potential implications for tumor progression. Oncoimmunology. 2013;2(2):e22946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Falanga A, Consonni R, Marchetti M, et al. Cancer procoagulant and tissue factor are differently modulated by all-trans-retinoic acid in acute promyelocytic leukemia cells. Blood. 1998;92(1):143–151. [PubMed] [Google Scholar]
- 103. Reinhardt C, von Bruhl ML, Manukyan D, et al. Protein disulfide isomerase acts as an injury response signal that enhances fibrin generation via tissue factor activation. J Clin. Invest. 2008;118(3):1110–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Langer F, Spath B, Fischer C, et al. Rapid activation of monocyte tissue factor by antithymocyte globulin is dependent on complement and protein disulfide isomerase. Blood. 2013;121(12):2324–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ahamed J, Versteeg HH, Kerver M, et al. Disulfide isomerization switches tissue factor from coagulation to cell signaling. Proc Natl Acad Sci USA. 2006;103(38):13932–13937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Rothmeier AS, Marchese P, Langer F, et al. Tissue factor prothrombotic activity is regulated by integrin-arf6 trafficking. Arterioscler Thromb Vasc Biol. 2017;37(7):1323–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Grignani G, Maiolo A. Cytokines and hemostasis. Haematologica. 2000;85(9):967–972. [PubMed] [Google Scholar]
- 108. Dosquet C, Weill D, Wautier JL. Cytokines and thrombosis. J Cardiovasc Pharmacol. 1995;25(Suppl 2):S13–S19. [DOI] [PubMed] [Google Scholar]
- 109. Puhlmann M, Weinreich DM, Farma JM, Carroll NM, Turner EM, Alexander HR., Jr Interleukin-1b induced vascular permeability is dependent on induction of endothelial tissue factor (TF) activity. J Transl Med. 2005;3:37–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Yamamura M, Yamada Y, Momita S, Kamihira S, Tomonaga M. Circulating interleukin-6 levels are elevated in adult T-cell leukaemia/lymphoma patients and correlate with adverse clinical features and survival. Br J Haematol. 1998;100(1):129–134. [DOI] [PubMed] [Google Scholar]
- 111. Zucchella M, Pacchiarini L, Meloni F, et al. Effect of interferon alpha, interferon gamma and tumor necrosis factor on the procoagulant activity of human cancer cells. Haematologica. 1993;78(5):282–286. [PubMed] [Google Scholar]
- 112. Mulloy B, Hogwood J, Gray E, Lever R, Page CP. Pharmacology of heparin and related drugs. Pharmacol Rev. 2016;68(1):76–141. [DOI] [PubMed] [Google Scholar]
- 113. Meyer G, Marjanovic Z, Valcke J, et al. Comparison of low-molecular-weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: a randomized controlled study. Arch Intern Med. 2002;162(15):1729–1735. [DOI] [PubMed] [Google Scholar]
- 114. Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl. J Med. 2003;349(2):146–153. [DOI] [PubMed] [Google Scholar]
- 115. Hull RD, Pineo GF, Brant RF, et al. Long-term low-molecular-weight heparin versus usual care in proximal-vein thrombosis patients with cancer. Am J Med. 2006;119(12):1062–1072. [DOI] [PubMed] [Google Scholar]
- 116. Lee AYY, Kamphuisen PW, Meyer G, et al. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA. 2015;314(7):677–686. [DOI] [PubMed] [Google Scholar]
- 117. Almarshad F, Alaklabi A, Bakhsh E, Pathan A, Almegren M. Use of direct oral anticoagulants in daily practice. Am J Blood Res. 2018;8(4):57–72. [PMC free article] [PubMed] [Google Scholar]
- 118. Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378(7):615–624. [DOI] [PubMed] [Google Scholar]
- 119. Young AM, Marshall A, Thirlwall J, et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D). J Clin Oncol. 2018;36(20):2017–2023. [DOI] [PubMed] [Google Scholar]
- 120. McBane II R, Loprinzi CL, Ashrani A, et al. Apixaban and dalteparin in active malignancy associated venous thromboembolism. The ADAM VTE trial. Thromb Haemost. 2017;117(10):1952–1961. [DOI] [PubMed] [Google Scholar]
- 121. Fuentes HE, McBane RD, 2nd, Wysokinski WE, et al. Direct oral factor Xa inhibitors for the treatment of acute cancer-associated venous thromboembolism: a systematic review and network meta-analysis. Mayo Clin. Proc. 2019;94(12):2444–2454. [DOI] [PubMed] [Google Scholar]
- 122. Agnelli G, Becattini C, Meyer G, et al. Apixaban for the treatment of venous thromboembolism associated with cancer. N Engl J Med. 2020;382(17):1599–1607. [DOI] [PubMed] [Google Scholar]
- 123. Stuijver DJ, van Zaane B, Romualdi E, Brandjes DP, Gerdes VE, Squizzato A. The effect of hyperthyroidism on procoagulant, anticoagulant and fibrinolytic factors: a systematic review and meta-analysis. Thromb Haemost. 2012;108(6):1077–1088. [DOI] [PubMed] [Google Scholar]
- 124. Chadarevian R, Bruckert E, Leenhardt L, Giral P, Ankri A, Turpin G. Components of the fibrinolytic system are differently altered in moderate and severe hypothyroidism. J. Clin. Endocrinol Metab. 2001;86(2):732–737. [DOI] [PubMed] [Google Scholar]
- 125. Salloum-Asfar S, Boelen A, Reitsma PH, van Vlijmen BJ. The immediate and late effects of thyroid hormone (triiodothyronine) on murine coagulation gene transcription. PLoS One. 2015;10(5):e0127469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Cristofanilli M, Yamamura Y, Kau SW, et al. Thyroid hormone and breast carcinoma. Primary hypothyroidism is associated with a reduced incidence of primary breast carcinoma. Cancer. 2005;103(6):1122–1128. [DOI] [PubMed] [Google Scholar]
- 127. Hercbergs AA, Goyal LK, Suh JH, et al. Propylthiouracil-induced chemical hypothyroidism with high-dose tamoxifen prolongs survival in recurrent high grade glioma: a phase I/II study. Anticancer res. 2003;23(1b):617–626. [PubMed] [Google Scholar]
- 128. Schmidinger M, Vogl UM, Bojic M, et al. Hypothyroidism in patients with renal cell carcinoma: blessing or curse? Cancer. 2011;117(3):534–544. [DOI] [PubMed] [Google Scholar]
- 129. Ashur-Fabian O, Blumenthal DT, Bakon M, Nass D, Davis PJ, Hercbergs A. Long-term response in high-grade optic glioma treated with medically induced hypothyroidism and carboplatin: a case report and review of the literature. Anticancer Drugs. 2013;24(3):315–323. [DOI] [PubMed] [Google Scholar]
- 130. Hercbergs A, Johnson RE, Fabian OA, Garfield DH, Davis PJ. Medically induced euthyroid hypothyroxinemia may extend survival in compassionate need cancer patients: an observational study. Oncologist. 2015;20(1):72–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Davis PJ, Goglia F, Leonard JL. Nongenomic actions of thyroid hormone. Nat Rev. Endocrinol. 2016;12(2):111–121. [DOI] [PubMed] [Google Scholar]
- 132. Lee YS, Chin YT, Shih YJ, et al. Thyroid hormone promotes beta-catenin activation and cell proliferation in colorectal cancer. Horm Cancer. 2018;9(3):156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Cayrol F, Diaz Flaque MC, Fernando T, et al. Integrin αvβ3 acting as membrane receptor for thyroid hormones mediates angiogenesis in malignant T cells. Blood. 2015;125(5):841–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Chang TC, Chin YT, Nana AW, et al. Enhancement by nano-diamino-tetrac of antiproliferative action of gefitinib on colorectal cancer cells: mediation by EGFR sialylation and PI3K activation. Horm Cancer. 2018;9(6):420–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Li W, Yalcin M, Bharali DJ, et al. Pharmacokinetics, biodistribution, and anti-angiogenesis efficacy of diamino propane tetraiodothyroacetic acid-conjugated biodegradable polymeric nanoparticle. Sci Rep. 2019;9(1):9006. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 136. Sudha T, Phillips P, Kanaan C, Linhardt RJ, Borsig L, Mousa SA. Inhibitory effect of non-anticoagulant heparin (S-NACH) on pancreatic cancer cell adhesion and metastasis in human umbilical cord vessel segment and in mouse model. Clin Exp Metastasis. 2012;29(5):431–439. [DOI] [PubMed] [Google Scholar]
- 137. Davis PJ, Sudha T, Lin HY, Mousa SA. Thyroid hormone, hormone analogs, and angiogenesis. Compr Physiol. 2015;6(1):353–362. [DOI] [PubMed] [Google Scholar]
- 138. Mousa SA, Bergh JJ, Dier E, et al. Tetraiodothyroacetic acid, a small molecule integrin ligand, blocks angiogenesis induced by vascular endothelial growth factor and basic fibroblast growth factor. Angiogenesis. 2008;11(2):183–190. [DOI] [PubMed] [Google Scholar]
- 139. Leith JT, Mousa SA, Hercbergs A, Lin H-Y, Davis PJ. Radioresistance of cancer cells, integrin αvβ3 and thyroid hormone. Oncotarget. 2018;9(97):37069–37075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Bishop GG, McPherson JA, Sanders JM, et al. Selective αvβ3-receptor blockade reduces macrophage infiltration and restenosis after balloon angioplasty in the atherosclerotic rabbit. Circulation. 2001;103(14):1906–1911. [DOI] [PubMed] [Google Scholar]
- 141. Mousa SA. Inhibitory effect of C-reactive protein on the release of tissue factor pathway inhibitor from human endothelial cells: reversal by low molecular weight heparin. Int Angiol. 2006;25(1):10–13. [PubMed] [Google Scholar]
- 142. Demers M, Krause DS, Schatzberg D, et al. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc Natl Acad. Sci USA. 2012;109(32):13076–13081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Cedervall J, Zhang Y, Huang H, et al. Neutrophil extracellular traps accumulate in peripheral blood vessels and compromise organ function in tumor-bearing animals. Cancer Res. 2015;75(13):2653–2662. [DOI] [PubMed] [Google Scholar]
- 144. Oehmcke S, Mörgelin M, Herwald H. Activation of the human contact system on neutrophil extracellular traps. J Innate Immun. 2009;1(3):225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Massberg S, Grahl L, von Bruehl ML, et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med. 2010;16(8):887–896. [DOI] [PubMed] [Google Scholar]
- 146. Hawes MC, Wen F, Elquza E. Extracellular DNA: A bridge to cancer. Cancer Res. 2015;75(20):4260–4264. [DOI] [PubMed] [Google Scholar]
- 147. Lewis HD, Liddle J, Coote JE, et al. Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nat Chem. Biol. 2015;11(3):189–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Gonzalez RD, Martínez-Colón GJ, Smith AJ, et al. Inhibition of neutrophil extracellular trap formation after stem cell transplant by prostaglandin E2. Am J Respir Crit Care Med. 2016;193(2):186–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Mohammed BM, Fisher BJ, Kraskauskas D, et al. Vitamin C: a novel regulator of neutrophil extracellular trap formation. Nutrients. 2013;5(8):3131–3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Khorana AA, McCrae KR. Risk stratification strategies for cancer-associated thrombosis: an update. Thromb Res. 2014;133(Suppl 2):S35–S38. [DOI] [PubMed] [Google Scholar]
- 151. Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111(10):4902–4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Ay C, Dunkler D, Marosi C, et al. Prediction of venous thromboembolism in cancer patients. Blood. 2010;116(24):5377–5382. [DOI] [PubMed] [Google Scholar]
- 153. Pabinger I, Thaler J, Ay C. Biomarkers for prediction of venous thromboembolism in cancer. Blood. 2013;122(12):2011–2018. [DOI] [PubMed] [Google Scholar]
- 154. Khorana AA. Risk assessment for cancer-associated thrombosis: what is the best approach? Thromb Res. 2012;129:S10–S15. [DOI] [PubMed] [Google Scholar]
- 155. Verso M, Agnelli G, Barni S, Gasparini G, LaBianca R. A modified Khorana risk assessment score for venous thromboembolism in cancer patients receiving chemotherapy: the Protecht score. Intern Emerg Med. 2012;7(3):291–292. [DOI] [PubMed] [Google Scholar]
- 156. Noble S, Pasi J. Epidemiology and pathophysiology of cancer-associated thrombosis. Br J Cancer. 2010;102(Suppl 1):S2–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Nuttall GA. Hemostasis and thrombosis: basic principles and clinical practice, 5th ed. Anesth Analg. 2007;104(5);1317. [Google Scholar]
- 158. Contrino J, Hair G, Kreutzer DL, Rickles FR. In situ detection of tissue factor in vascular endothelial cells: correlation with the malignant phenotype of human breast disease. Nat Med. 1996;2(2):209–215. [DOI] [PubMed] [Google Scholar]
- 159. Rak J. Microparticles in cancer. Semin Thromb Hemost. 2010;36(8):888–906. [DOI] [PubMed] [Google Scholar]
- 160. Versteeg HH, Ruf W. Emerging insights in tissue factor-dependent signaling events. Semin Thromb Hemost. 2006;32(1):24–32. [DOI] [PubMed] [Google Scholar]
- 161. Fischer EG, Riewald M, Huang HY, et al. Tumor cell adhesion and migration supported by interaction of a receptor-protease complex with its inhibitor. J Clin Invest. 1999;104(9):1213–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Bieker R, Kessler T, Schwoppe C, et al. Infarction of tumor vessels by NGR-peptide-directed targeting of tissue factor: experimental results and first-in-man experience. Blood. 2009;113(20):5019–5027. [DOI] [PubMed] [Google Scholar]
- 163. van den Berg YW, Osanto S, Reitsma PH, Versteeg HH. The relationship between tissue factor and cancer progression: insights from bench and bedside. Blood. 2012;119(4):924–932. [DOI] [PubMed] [Google Scholar]