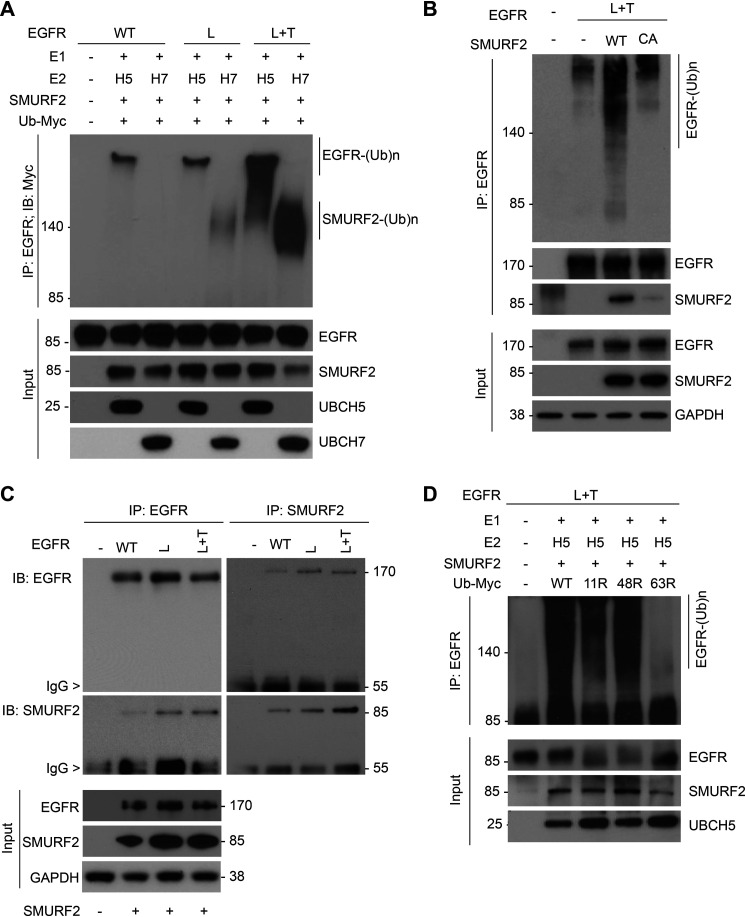
Figure 1.
EGFR (L858R/T790M) is a preferred substrate for SMURF2-UBCH5-mediated ubiquitination. A, purified EGFR proteins, either WT or mutants (L858R [L] and L858R/T790M [L+T]), were subjected to in vitro ubiquitination using recombinant SMURF2 as an E3 in the presence of either UBCH5 or UBCH7 as E2 enzymes. Following completion, reaction mixtures were subjected to immunoprecipitation using the EGFR antibody followed by immunoblotting using the indicated antibodies. B, CHO cells overexpressing (L+T) mutant EGFR alone or in the presence of WT or catalytic-dead (C716A) SMURF2 were immunoprecipitated with EGFR antibody followed by immunoblotting using the indicated antibodies. C, CHO cells, either vector control (−) or overexpressing EGFR (WT, L, and L+T), and SMURF2 were immunoprecipitated with either EGFR or SMURF2 antibodies and immunoblotted for the indicated antibodies. D, SMURF2-UBCH5-mediated in vitro ubiquitination of mutant EGFR (L+T) was performed as described above in the presence of WT or different ubiquitin mutants (K11R, K48R, and K63R) deficient in promoting specific linkages. Higher-molecular-weight ubiquitinated EGFR species were detected following immunoprecipitation and immunoblotting.