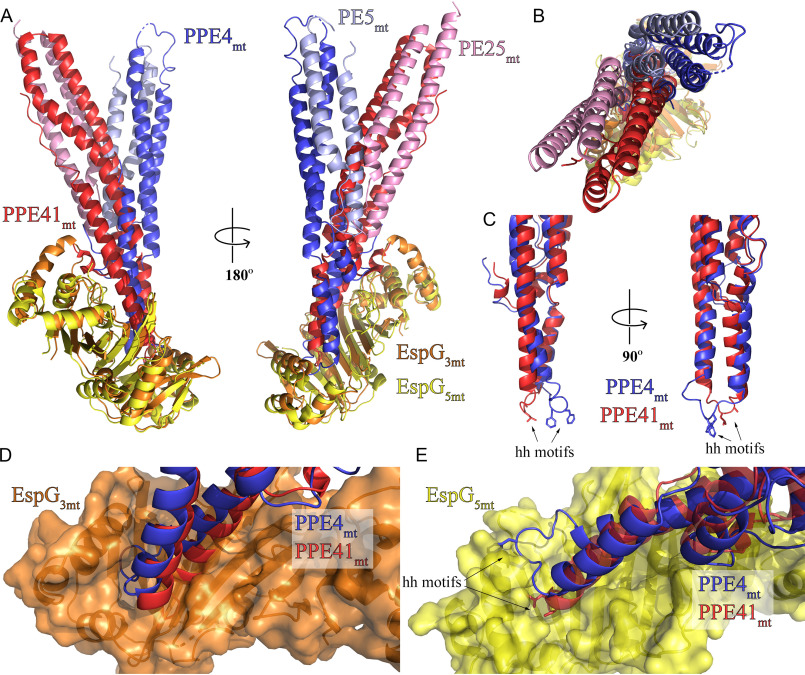
Figure 5.
PE5mt–PPE4mt interacts with EspG3mm chaperone in a unique mode compared with ESX-5 PE–PPE dimers. A, structural alignment of the ESX-3 and ESX-5 heterotrimers via the EspG chaperones (36) reveals a difference in the angle of interaction between the PE–PPE heterodimers with their respective chaperone. B, top view of alignment from A. C, superposition of PPE41mt and PPE4mt highlights difference in hh loop conformations between ESX-3 (PPE4mt) and ESX-5 (PPE41mt). D and E, superposition of PPE alignment from C in context of EspG3mm interaction (D) and EspG5mt interaction (E) shows the incompatibility of each PPE protein with noncognate chaperone binding.