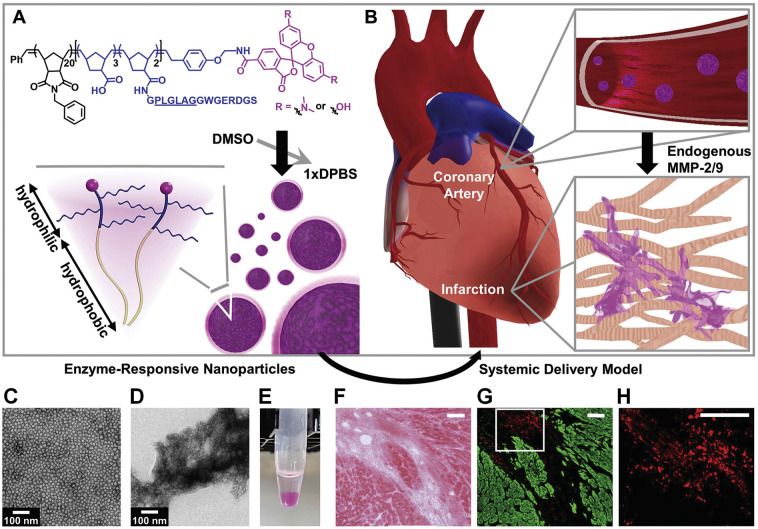
Fig. 3.
Enzyme-responsive nanoparticles for targeted accumulation and prolonged retention in heart tissue after myocardial infarction (MI). (A) Diagram of a dye-labeled brush peptide-polymer amphiphile (PPA) containing an MMP-2 and MMP-9 specific recognition sequence, shown underlined. PPAs self-assemble into nanoparticles through hydrophobic-hydrophilic interactions when dialyzed into aqueous buffer. (B) Schematic of nanoparticles freely circulating in the bloodstream upon systemic delivery. Nanoparticles enter the infarct tissue through the leaky acute MI vasculature, and up-regulated MMPs within the infarct induce the formation of an aggregate-like scaffold. (C) Responsive nanoparticles are monodisperse micelles with diameters of 15–20 nm. (D) Formation of an aggregate-like scaffold upon activation of responsive nanoparticles. (E) A corresponding image of nanoparticle solutions following activation. (F-H) Retention of responsive nanoparticles upon localized delivery. Particles were injected intramyocardially at day 7 post-MI and assessed at day 6 postinjection. (F) H&E image displays the infarct area. (G) The neighboring fluorescent section. Particles are shown in red and myocardium is shown in green. (H) A selected region from (G) (white outline) was magnified to highlight particle aggregation. Scale bars in (F-H), 100 μm. Reproduced with permission [288]. Copyright 2015, Wiley-VCH.