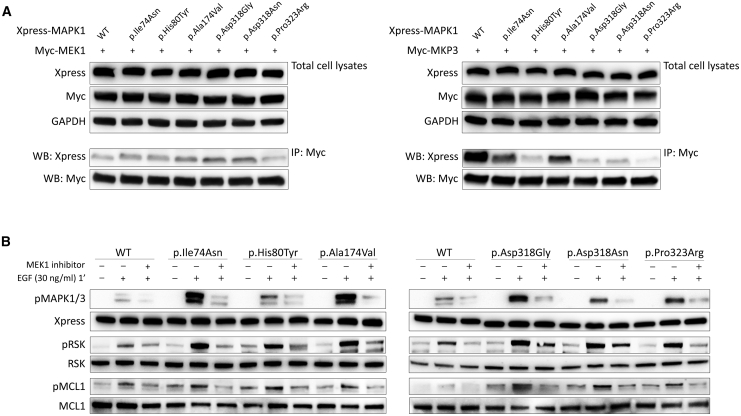
Figure 6.
Disease-Causing Mutations Impair Binding of MAPK1 with MKP3 but Not Binding to MEK1, and Retain Dependence on MEK Activity in Their Upregulation of MAPK Signaling
(A) Co-immunoprecipitation assays. Lysates from HEK293T cells transiently transfected to express wild-type and mutant Xpress-tagged MAPK1 protein with Myc-MEK1 or Myc-MKP3 were immunoprecipitated with an anti-Myc antibody and assayed by western blotting using the indicated antibodies.
(B) MAPK1 mutation-promoted MAPK signal upregulation retains dependence on MEK activity. MAPK, RSK, and MCL1 phosphorylation assays were performed in transiently transfected HEK293T cells starved for 16 h and stimulated with EGF (30 ng/mL, 1 min) after treatment with the MEK inhibitor trametinib (1.5 ng/mL for 2 h). Blots show a decrease in pMAPK, pRSK and pMCL1 in cells overexpressing wild-type or mutant MAPK1 proteins in presence of trametinib, indicating that the mutation-driven signal upregulation of the MAPK cascade retains dependence on MEK activity.