Abstract
Necrotizing sialometaplasia (NSM) is a benign, reactive metaplastic condition of the minor salivary gland tissue typically seen in the setting of injury, chemical or traumatic, and is nonneoplastic and self-limited. The diagnosis may be challenging as it may clinically mimic malignancy. We present the case of a 74-year-old male with a 1 pack per day smoking history for 60 years who presented with a reported 20-pound weight loss, dysphagia, and dysphonia progressing over the course of 6 months and found to have a 3.5-cm hypopharyngeal mass on computed tomography imaging and fiberoptic laryngoscopy. Initial frozen section of the mass was concerning for squamous cell carcinoma in situ, but permanent specimens returned as nondiagnostic. Repeat biopsy established a diagnosis of NSM. Two-month follow-up showed complete resolution of the mass. Clinicians should be aware that NSM may present in unusual locations when considering differential diagnoses for laryngeal masses and evaluating for malignancy.
Keywords: necrotizing sialometaplasia, hypopharynx, malignancy, salivary gland malignancy, head and neck cancer
Introduction
Necrotizing sialometaplasia (NSM) may be clinically and histopathologically difficult to distinguish from malignancy. Necrotizing sialometaplasia most frequently occurs in the hard palate but can occur in other subsites of the oral cavity and rarely extends to other portions of the airway, anywhere minor salivary gland tissue may be present.1,2 Males are twice as likely to be affected as females and the average age of onset is 46 years old.1 Often a secondary pathologic finding rather than a primary cause for clinical presentation, local inflammatory changes and possibly ischemia may be associated with the evolution of NSM, with smoking and local, chronic trauma associated with onset.3,4 In this case, we describe NSM presenting clinically as a hypopharyngeal mass.
Methods and Materials
This study was approved under IRB 966570–3. A review of a cases was performed through PubMed using the search term “necrotizing sialometaplasia” in the English language. A total of 147 articles were identified, with 131 found to be appropriate after abstract review. Of these 131 articles, 110 were case reports, 18 were case series, and 3 were literature reviews. None of these articles described isolated NSM occurring in the hypopharynx as was seen in this case.
Case Report
The patient is a 74-year-old male with a 1 pack per day smoking history for 60 years who presented with a reported 20-pound weight loss, dysphagia, and dysphonia progressing over the course of 6 months, the weight loss occurring in the absence of effort. On initial evaluation in the emergency department, he was noted to have a rightward anterior deviation of the arytenoid complex with a hypomobile right vocal fold. There appeared to be a posterior commissure thickening imposing on the glottic airway. A subsequent computed tomography scan (Figure 1) showed a 3.5 cm × 1.8 × cm 2.1 cm mass in the hypopharynx with mass effect displacing the glottic airway anteriorly. Given the high likelihood of malignancy, the patient was scheduled for direct laryngoscopy and biopsy of the hypopharyngeal mass, as well as gastrostomy tube placement. From the operating room, an initial frozen section of the right postcricoid mass was concerning for squamous cell carcinoma in situ and specimens were sent for permanent pathology. Multidisciplinary evaluation of this patient with presumed T3N0M0 squamous cell carcinoma of the hypopharynx yielded a consensus recommendation for upfront surgery, including total laryngopharyngectomy and bilateral neck dissections, including a free flap reconstruction with postoperative adjuvant treatment pending for the final pathologic examination of the biopsy material.
Figure 1.
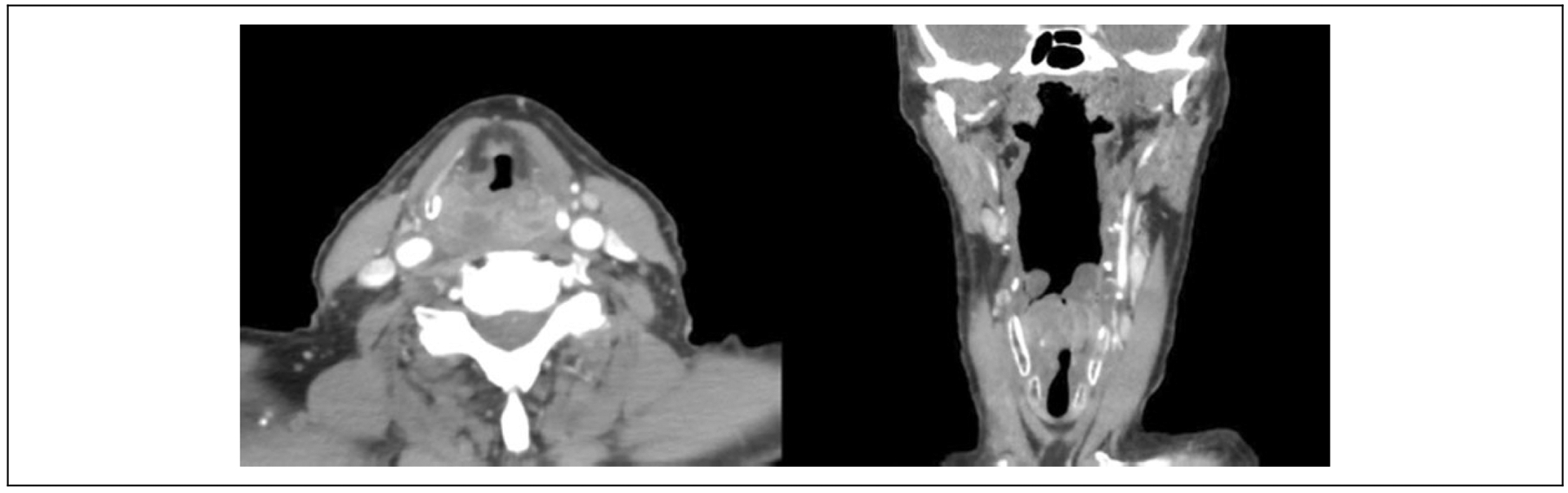
Computed tomography scan of hypopharyngeal mass showing right-sided 3.5-cm partially cystic mass, with mass effect displacing glottic airway anteriorly.
Final review by the pathologist revealed hyperplasia of the tissue surface best noted in Figure 2A, with thickened cords of squamous epithelium and underlying mildly irregular nests of cells in an organized pattern lacking dysplastic features or atypical mitotic figures most consistent with necrotizing sialometaplasia. There was evidence of possible previous trauma with fibrin (Figure 2A, arrowhead; Figure 2B, asterisks) pooling adjacent to the hyperplastic epithelium (Figure 2B, arrows). Additionally, there was stromal edema and frank ulceration (Figure 2C) with necroinflammatory debris. There was no evidence of neoplasia.
Figure 2.

Necrotizing sialometaplasia of hypopharyngeal mass. Sialometaplasia (A): Hyperplastic squamous mucosa with sialometaplasia (arrows) and fibrin deposition (arrow head), ×100 (H&E stain). Hyperplasia (B): Hyperplastic squamous mucosa (arrows) with pronounced underlying fibrin deposition (asterisks) consistent with hemorrhage (H&E stain, ×100). Ulcer (C): Ulcer bed (H&E) with entirely necroinflammatory debris. No viable tissue seen (right posterior cricoid, ×200).
Given the highly unusual location of the finding and significant ramifications of diagnosis, the patient returned to the operating room for a second direct laryngoscopy and biopsy. During the second direct laryngoscopy, the mass was noted to have decreased in size and have less ulceration than noted on initial examination. The pathologist’s final evaluation was reported as hyperplastic squamous mucosa with chronic inflammation and granulation tissue lacking evidence of carcinoma or dysplasia. The patient was discharged home with monthly follow-up to monitor for resolution of necrotizing sialometaplasia, given the highly unusual location of the mass. Two-month follow-up showed complete resolution of the mass, the patient tolerating a full diet with removal of gastrostomy tube after sustained weight gain, and resolution of underlying dysphagia.
Discussion
Necrotizing sialometaplasia is a nonneoplastic lesion presenting with metaplasia of the salivary gland ducts and acini and resultant ischemic necrosis of the ductal systems and acinar lobules, often with intraductal necroinflammatory debris. The presumed pathogenesis is traumatic in nature, either chemical or mechanical, with evolution involving local inflammatory and ischemic change. Contributing factors may include smoking, alcohol, radiation, and chronic trauma, as in the setting of ill-fitting dentures or prostheses. Presenting clinical features of this disease are nonspecific and depend on the size and location of the lesion. In most cases, NSM presents as a secondary finding associated with its inciting event, trauma, or inflammation (which may be a result of infection or neoplasia). Fevers, myalgias, and localized pain have been associated with NSM. The differential diagnosis of NSM is broad, including malignant and benign entities. Among malignancies, squamous cell carcinoma is the primary differential, a diagnostic pitfall if biopsy material is limited. Also in the differential are adeno-carcinoma, mucoepidermoid carcinoma, and metastasis. Among underlying inflammatory etiologies in this location, infectious lesions would be more common and include syphilis or tuberculosis. As mentioned, further confounding the diagnosis of NSM is that it may occur in association with an underlying malignancy, and thus, a thorough workup for malignancy is mandatory if the clinical picture remains suspicious in the setting of NSM.2,5
Necrotizing sialometaplasia is a pathologic diagnosis, with preservation of salivary gland lobular architecture in the setting of intraductal necrosis and an inflammatory background (Figure 2). The sialometaplasia is squamatization of glandular structures associated with squamous metaplasia of ductal and acinar epithelium. It is typically found as a reactive process alongside inflammation for other reasons (including adjacent to ulcer bed, associated with a chronic or acute inflammatory process, or adjacent to a neoplasia). Sialometaplasia, itself, is a reactive, metaplastic process and nonneoplastic. Depending on when in its course the NSM is biopsied, necrosis and ulceration may be present in varying degrees, with preservation of lobular architecture.6 Staining can aid in diagnosis with immunohistochemistry showing low p53 expression and low to absent MIB1 (Ki-67) proliferative activity. Calponin or p63 may highlight myoepithelial cells and keratin stains epithelial cells to establish the bilayered nature of the salivary tissue, although this is typically not needed nor necessary for diagnosis.4
Of notable interest in this case is the location and size of the NSM. As a disease of the minor salivary glands, NSM most commonly occurs in the palate (80% prevalence) and oral cavity.1 The posterior hard palate is the most common location with the junction between the hard and soft palate as the second most common site.1 In these locations, a simple incisional biopsy can lead to a tissue diagnosis. Lesions more typically occur individually but have been noted to occur as multiple lesions and bilaterally as well.7 Estimates of typical size of NSM lesions vary greatly, with masses as large as 5 cm being reported in the literature, but generally these lesions present as shallow ulcerations of the palate.1,6
While NSM can theoretically occur at any location where minor salivary gland tissue exists, to our knowledge, this is the first description of NSM occurring in the hypopharynx. While typical NSM lesions of the palate can be easily biopsied, obtaining tissue for diagnosis of the hypopharynx is significantly more involved. Moreover, the large size (3.5 cm) of the mass in conjunction with smoking, weight loss, voice changes, and difficulty swallowing made this case particularly concerning for malignancy. The importance of the definitive histopathology in this case cannot be overstated. Although initial frozen sections were suggestive of carcinoma, especially when the pathologist is presented with a clinical suspicion of malignancy compounded by the irregularity of the hyperplastic epithelium, the lack of conclusive evidence of malignancy in the biopsy avoided the potential extensive surgery (ie, laryngopharyngectomy) for which the patient was scheduled. Significantly, this case illustrates the importance of thorough tissue sampling and a conclusive diagnosis of malignancy even when faced with a clinical scenario that is suggestive of cancer. Fortunately, our patient demonstrated complete resolution of NSM. Given the unusual presentation and rarely observed evidence that NSM can recur,8,9 he will require close monitoring.
Conclusion
Necrotizing sialometaplasia is known to mimic malignancy and may result in inappropriately aggressive treatment. A condition that most commonly affects the oral cavity, this case demonstrates NSM presenting as a clinically significant mass in the hypopharynx that was concerning for malignancy. Clinicians should be aware that NSM may present in unusual locations when considering differential diagnoses for laryngeal masses and evaluating for malignancy.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Brannon RB, Fowler CB, Hartman KS. Necrotizing sialometaplasia. A clinicopathologic study of sixty-nine cases and review of the literature. Oral Surg Oral Med Oral Pathol. 1991;72(3):317–325. [DOI] [PubMed] [Google Scholar]
- 2.Ravn T, Trolle W, Kiss K, Balle VH. Adenosquamous carcinoma of the larynx associated with necrotizing sialometaplasia—a diagnostic challenge. Auris Nasus Larynx. 2009;36(6):721–724. doi: 10.1016/j.anl.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Joshi SA, Halli R, Koranne V, Singh S. Necrotizing sialometaplasia: a diagnostic dilemma! J Oral Maxillofac Pathol JOMFP. 2014; 18(3):420–422. doi: 10.4103/0973-029X.151336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlson DL. Necrotizing sialometaplasia: a practical approach to the diagnosis. Arch Pathol Lab Med. 2009;133(5):692–698. doi: 10.1043/1543-2165-133.5.692. [DOI] [PubMed] [Google Scholar]
- 5.Poulson TC, Greer RO, Ryser RW. Necrotizing sialometaplasia obscuring an underlying malignancy: report of a case. J Oral Maxillofac Surg. 1986;44(7):570–574. [DOI] [PubMed] [Google Scholar]
- 6.Krishna S, Ramnarayan BK. Necrotizing sialometaplasia of palate: a case report. Imaging Sci Dent. 2011;41(1):35–38. doi: 10.5624/isd.2011.41.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keogh PV, O’Regan E, Toner M, Flint S. Necrotizing sialometaplasia: an unusual bilateral presentation associated with antecedent anaesthesia and lack of response to intralesional steroids. Case report and review of the literature. Br Dent J. 2004;196(2):79–81. doi: 10.1038/sj.bdj.4810892. [DOI] [PubMed] [Google Scholar]
- 8.Close LG, Cowan DF. Recurrent necrotizing sialometaplasia of the nasal cavity. Otolaryngol-Head Neck Surg. 1985;93(3):422–425. doi: 10.1177/019459988509300326. [DOI] [PubMed] [Google Scholar]
- 9.Jeong CW, Youn T, Kim HS, Park KH, Huh JK. Contralateral recurrence of necrotizing sialometaplasia of the hard palate after five months: a case report. J Korean Assoc Oral Maxillofac Surg. 2015;41(6):338–341. doi: 10.5125/jkaoms.2015.41.6.338. [DOI] [PMC free article] [PubMed] [Google Scholar]


