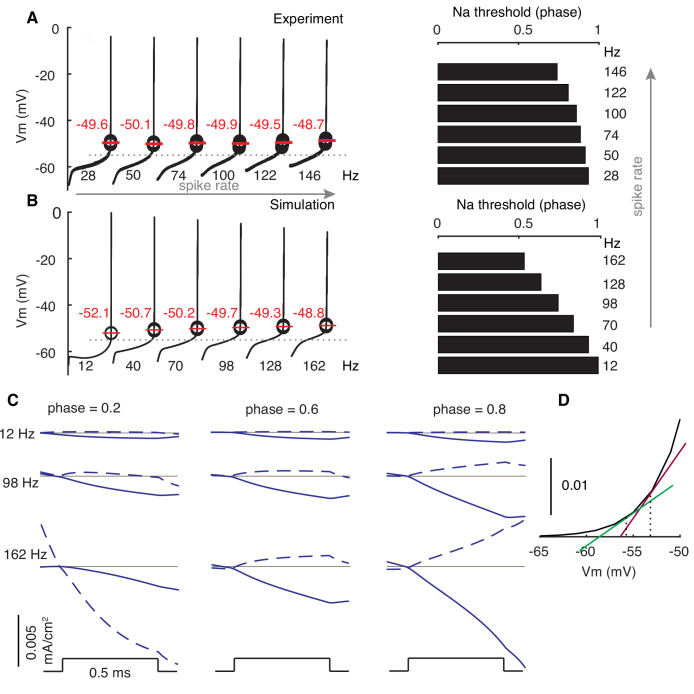
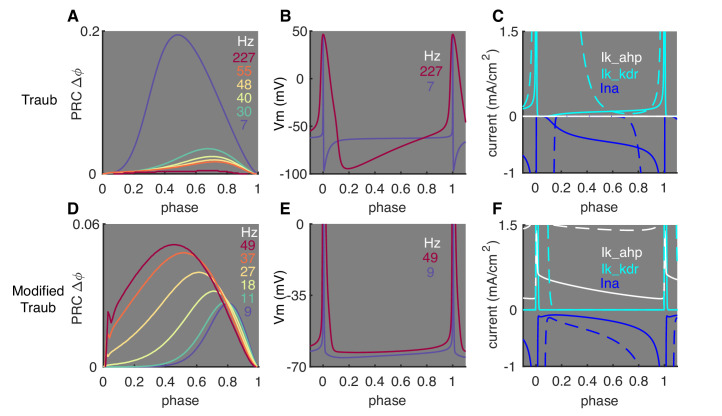
We examined whether the critical role of subthreshold membrane potentials in shaping PRC profiles (
Figure 2) also applies to other neuron types. A frequently used pyramidal neuron model, the Traub model (
Ermentrout et al., 2001) was tested. It shows an opposite rate adaptation of PRCs compared to PCs (
A). In the Traub model, responses become smaller and relatively phase-independent at high firing rates. This demonstrates that the normalization used in
Equation 1 does not always lead to increasing PRC peak amplitudes for smaller ISIs. These PRC shapes can be explained by significantly lower subthreshold membrane potentials at high rates, compared to PCs, (
B). This is due to the accumulation of delayed rectifier K
+ current (kdr,
C), which has a low activation threshold and large conductance. The lower subthreshold membrane potentials are far below the Na
+-activation threshold, making responses to weak stimuli passive at high firing rates. Accordingly, PRCs in the model become smaller and relatively phase-independent at high rates. We minimally modified the Traub model by reducing the conductance of the kdr current, raising its activation threshold and increasing the AHP current (details in Materials and methods) (
D-F). With these modifications, subthreshold membrane potentials are significantly elevated at high firing rates lower than 110 Hz. Accordingly, onset-phases of phase-dependent responses shift left and peaks increase at high rates. These simulation results show that spike rate-dependent subthreshold membrane potentials and their effect on nonlinear activation of Na
+ currents can be crucial in shaping neuronal PRC profiles in many types of neurons. Surprisingly, when firing rates are higher than 110 Hz, PRCs decrease again, suggesting other undetermined biological mechanisms may be involved in determining the phase responses. (
A) Rate adaptation of PRCs in the original Traub model. (
B) Lowered ISI membrane potential at high rates. (
C) Comparison of ionic currents at low (solid, 7 Hz) and high (dashed, 227 Hz) rates. (
D) Rate adaptation of PRCs in the modified Traub model. (
E) Elevated ISI membrane potential at high rates. (
F) Comparison of ionic currents at low (solid, 9 Hz) and high (dashed, 49 Hz) rates. In (
C) and (
F), current peaks are truncated to show currents during ISIs. In (
E), spike peaks are truncated to show the elevated ISI membrane potential at high rates.