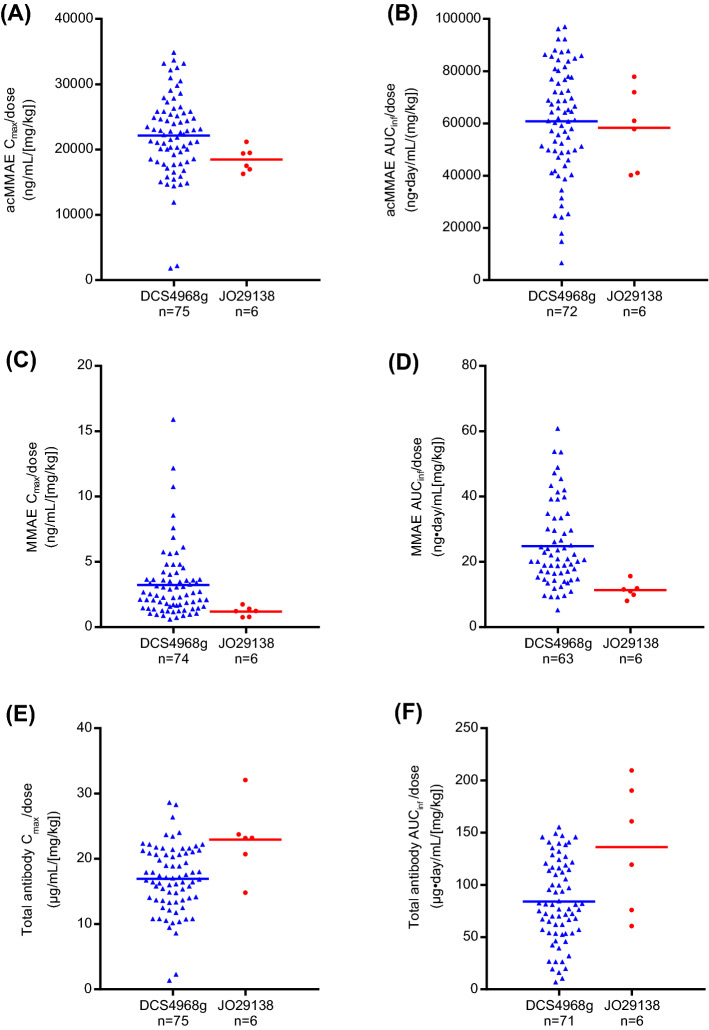
Fig. 1.
a Dose-normalized Cmax for acMMAE; (b) dose-normalized AUCinf for acMMAE; (c) dose-normalized Cmax for MMAE; (d) dose-normalized AUCinf for MMAE; (e) Dose-normalized Cmax for total antibody; (f) dose-normalized AUCinf for total antibody in Cycle 1 in DCS4968g (NCT01290549) and JO29138 (JAPICCTI‐142580) studies following pola monotherapy, by NCA. Blue triangles represent individual patients from study DCS4968g (NCT01290549; 0.1–2.4 mg/kg), and red circles represent individual patients from study JO29138 (JAPICCTI‐142580; 1.0–1.8 mg/kg). Horizontal lines represent the mean values. acMMAE antibody-conjugated monomethyl auristatin E, AUCinf area under the concentration–time curve from time 0 to infinity, Cmax maximum concentration observed, MMAE monomethyl auristatin E, pola polatuzumab vedotin